Abstract
Inadvertent social information (ISI) use, i.e., the exploitation of social cues including the presence and behaviour of others, has been predicted to mediate population-level processes even in the absence of cohesive grouping. However, we know little about how such effects may arise when the prey population lacks social structure beyond the spatiotemporal autocorrelation originating from the random movement of individuals. In this study, we built an individual-based model where predator avoidance behaviour could spread among randomly moving prey through the network of nearby observers. We qualitatively assessed how ISI use may affect prey population size when cue detection was associated with different probabilities and fitness costs, and characterised the structural properties of the emerging detection networks that would provide pathways for information spread in prey. We found that ISI use was among the most influential model parameters affecting prey abundance and increased equilibrium population sizes in most examined scenarios. Moreover, it could substantially contribute to population survival under high predation pressure, but this effect strongly depended on the level of predator detection ability. When prey exploited social cues in the presence of high predation risk, the observed detection networks consisted of a large number of connected components with small sizes and small ego networks; this resulted in efficient information spread among connected individuals in the detection networks. Our study provides hypothetical mechanisms about how temporary local densities may allow information diffusion about predation threats among conspecifics and facilitate population stability and persistence in non-grouping animals.
Significance statement
The exploitation of inadvertently produced social cues may not only modify individual behaviour but also fundamentally influence population dynamics and species interactions. Using an individual-based model, we investigated how the detection and spread of adaptive antipredator behaviour may cascade to changes in the demographic performance of randomly moving (i.e., non-grouping) prey. We found that social information use contributed to population stability and persistence by reducing predation-related per capita mortality and raising equilibrium population sizes when predator detection ability reached a sufficient level. We also showed that temporary detection networks had structural properties that allowed efficient information spread among prey under high predation pressure. Our work represents a general modelling approach that could be adapted to specific predator-prey systems and scrutinise how temporary local densities allow dynamic information diffusion about predation threats and facilitate population stability in non-grouping animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organisms have to gather information about their surroundings to overcome challenges such as finding resources and avoiding danger (Dall and Johnstone 2002). For that, individuals directly interact with the environment to gain up-to-date information about its state (‘personal information’), but they can also complement that knowledge by utilising social information for optimal decision-making (Galef and Giraldeau 2001; Dall et al. 2005; Bonnie and Earley 2007; Hoppitt and Laland 2013). One type of social information is associated with inadvertently produced social cues that include the presence or the behaviour of others, or the product of their behaviour such as scent marks, excretions or food remnants, all of which may provide relevant information about current environmental conditions. Inadvertent social information (ISI) use is known to occur in many ecological contexts, including predator avoidance, foraging and habitat choice (Danchin et al. 2004; Gil et al. 2018). The advantages of living in social groups are thought to include the opportunity to access social information (Krause and Ruxton 2002; Ward and Webster 2016; Goodale et al. 2017), and thus ISI use is usually associated with species where social interactions promote information transmission among groupmates (King and Cowlishaw 2007; Duboscq et al. 2016; Gil et al. 2017).
Under predation risk, dynamic information about threats is transmitted from alarmed group members to naïve ones, a phenomenon that is commonly called collective detection (Lima 1990; Pays et al. 2013). This process often takes place through evolved signals such as alarm calls, but social cues including sudden movements (Coleman 2008; Hingee and Magrath 2009; Boujja-Miljour et al. 2017), fright responses (Chivers and Ferrari 2014; Cruz et al. 2020) or changes in posture (Brown et al. 1999; Pays et al. 2013) have also been found to convey information about the presence of predators in animal collectives. Adjustments to the behaviour of others (also referred to as ‘behavioural contagion’; Firth 2020) do not only affect individual fitness by increasing survival probabilities, but can also lead to the emergence of correlated behaviours and space use in many individuals and thus influence system-level functions (Goodale et al. 2010; Gil et al. 2018; Tóth 2021). Previous theoretical models have predicted that ISI use can prevent population collapses under high predation pressure (Gil et al. 2017, 2018) and facilitate the coexistence of competing species that share common predators (Parejo and Avilés 2016; Gil et al. 2019). Empirical evidence also indicates that the utilisation of social information can influence the material flux on the ecosystem level (Gil and Hein 2017). By promoting adaptive behavioural responses to environmental uncertainties (e.g., due to anthropogenic effects (Greggor et al. 2017), in the distribution of resources (O’Mara et al. 2014) or predation risk (Crane et al. 2022)), ISI use has the potential to minimise the impact of morphological, physiological or genetic adaptations (Laland 1992) or influence genetic change through gene–culture coevolution (Whitehead et al. 2019).
There are animal species that do not exhibit social attraction toward conspecifics and therefore do not form permanent or periodical cohesive groups. We refer to these organisms as non-grouping animals (for more details about to this definition, see Tóth et al. 2020). Lacking motivation for social cohesion, non-grouping animals do not maintain spatial proximity with others, and thus direct interactions between conspecifics can be infrequent. Nevertheless, such individuals may also exploit social cues (e.g., visual, acoustic, chemical or vibrational cues) when these are within the range of relevant sensory perception. Moreover, social information may also diffuse among nearby observers via ‘detection networks’ (reviewed in Tóth et al. 2020). If so, spatial changes in social cues over time (e.g., relative differences in activity and associated conspicuousness; Chivers and Ferrari 2014) can provide dynamic information about predation threats in many terrestrial and aquatic systems (Gil et al. 2017). In accordance with this idea, wood crickets (Nemobius sylvestris) adaptively change their behaviour after having observed the predator avoidance behaviour of knowledgeable conspecifics, and this information is transmitted to and utilised by other naïve individuals as well (Coolen et al. 2005). In temporary aggregations, escape responses of Iberian green frogs (Rana perezi) are also influenced by the behaviour of adjacent conspecifics (Martín et al. 2006). In mixed-species aggregations of non-schooling fish, the density (number of fish in the foraging area) and behaviour (when to feed in and when to flee from the foraging area) of nearby individuals are being used as inadvertent social information (Gil and Hein 2017). The resulting behavioural coupling among individuals, in turn, affects both species abundance and the amount of algae consumed and as a result, determines the total material flow in the coral reef ecosystem. While such observations prove that threat-related social cues can be exploited by non-grouping animals in some instances, the general conditions under which ISI use exerts a positive effect on population stability and persistence in such species have remained largely unexplored. For example, thresholds associated with the cost of antipredator behaviour and probabilities of cue detection (i.e., the detection of predators or conspecifics’ behaviour) may set boundaries for social information-modulated population-level effects under different predation pressure regimes. Similarly, detection networks may have only a limited capacity to provide efficient information pathways for the emergence of such effects. In a previous work, Tóth (2021) used an individual-based model to test specifically how ISI use may alter the relationship between fluctuating predator and non-grouping prey populations. The author found that ISI use can disrupt population cycles and decrease the strength of second-order density dependence between predator and prey (i.e., negative feedback of the second order that would imply an interaction between predator and prey populations in either direction), thus stabilize their dynamics and facilitate their long-term coexistence.
In this study, we investigated how the detection and spread of predator avoidance behaviour among conspecifics affected demographic performance in non-grouping prey. We constructed an individual-based model of prey and generalist predator populations where individuals (both prey and predators) moved randomly on the landscape, and social information could diffuse through the observation of antipredator behaviour in prey. This model, an extension of our earlier model presented by Tóth (2021), allowed us to assess qualitatively how ISI use may cascade to population-level changes in noncyclic prey populations that experience relatively constant predation pressures and lack social structure. We could also examine the structural properties of detection networks that might facilitate the emergence of such effects. Thus, predictions from the presented model may be applied more generally to non-grouping organisms compared to those from our previous work.
Materials and methods
Model construction
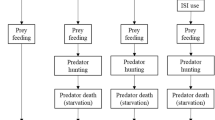
We simulated a homogeneous, continuous 2D landscape (80 × 80 spatial units) where both prey and predators moved randomly by exhibiting correlated random walks (CRW). CRW considers short-term correlations between successive step orientations and has been successfully used to model animals’ non-orientated search paths for a long time (Benhamou 2006; Codling et al. 2008; Reynolds 2014). In CRW models, habitat must be rather homogeneous; examples of such natural habitats include beaches and deserts, grasslands, agricultural crops or those where resource patch distribution at the large scale is uniform or random (Byers 2001). At the start of a simulation cycle, 500 prey and 150 predators were randomly placed on the landscape, and then individuals performed a given set of behaviours (Fig. 1, Table 1). During movement, each individual’s movement distance was randomly selected between zero and a maximum value given by the parameters dprey and dP for prey and predators, respectively. Turning angles were determined by random deviates drawn from wrapped Cauchy circular distribution with μ = 0 and ρ = 0.8. At the landscape edge, individuals moved to the opposite side of the landscape when crossing a boundary and continued moving (i.e., torus landscape with no edge). Both prey and predator could also detect other individuals through the landscape edge. We assumed that only one individual could survive within the range of one spatial unit due to competition in both prey and predators (after movement and dispersion of offspring; see Fig. 1), introducing density-dependent mortality in their populations. In this system, we assumed non-dynamic predators that can exert high predation pressure on the prey population, thus predator population size was determined only by their reproductive rate and density-dependent mortality, but was unaffected by the success of hunting (as if switching to alternative prey when necessary). Consequently, predator and prey populations were noncyclic and demographically decoupled (for a similar approach, see Gil et al. 2019), and prey populations experienced predation pressures that were directly proportional to the given value of predators’ reproduction-related parameter (Table 1).
Model flowchart for a single simulation cycle. Sequential prey and predator behaviours are listed together with the model parameter(s) associated with the given steps. Behavioural steps resulting in a decrease in population size, i.e., mortality due to intraspecific competition (in rounded rectangles) or predation (in diamond) are shown in light and dark grey, respectively
In the absence of predators, prey moved, competed, fed and reproduced in the simulated landscape. Prey population size resulted in this scenario was regarded as being in equilibrium at the carrying capacity of the environment. When present, each predator could consume a maximum of five prey individuals in a cycle within its hunting range, which was defined as an rP distance from the predator’s position in any direction. Prey could detect predators that were rprey distance with a probability given by Pdetect (determined by individual Bernoulli trials). Thus, the detection of other individuals (either predators, prey or conspecifics) depended on individual sensory ranges that also crossed through the torus landscape edges, bringing more reality to the simulation compared to the model presented in Tóth (2021). Upon successfully detecting a predator within rprey distance, prey became alarmed and hid, and thus were undetectable to predators. However, these individuals did not feed either and consequently could have a reduced reproduction rate. Thus, prey animals were capable of behaviourally adjusting their exposure to predators (with the probability ranging between 0.1 and 0.9; see Table 1), but this antipredator behaviour potentially incurred a fitness cost. Lima and Dill (1990) summarized supporting evidence for costly behavioural responses to predation risk in multiple taxa, including a reduction in feeding, growth or reproduction. Predators hunted on visible, feeding prey with a 50% success (determined by individual Bernoulli trials). Similarly high success rates against some prey species have been observed in generalist predators such as red foxes (Vulpes vulpes; Červený et al. 2011), Drassodes lapidosus spiders (Michálek et al. 2017) or American kestrels (Falco sparverius; Toland 1987). Prey could also detect predators indirectly by observing alarmed conspecifics within rprey distance with a probability given by Pisi (determined by individual Bernoulli trials). Being alarmed had the same consequences (i.e., immune to predation, reduced reproduction rate) irrespective of the detection mode. Wide ranges of possible values for both Pdetect and Pisi (see in Table 1) were used to cover most scenarios in which ISI use may occur under natural conditions. We did not manipulate cue reliability in the model, we simply considered that ISI use had a higher cost when social cues could also be false and individuals responded to those indiscriminately. Prey feeding occurred once in a cycle in prey that was not hiding. The number of offspring for each individual was sampled from a Poisson distribution with the shape parameter given by λreduced for alarmed prey, λmax for fed prey and λP for predators in each cycle. Offspring dispersed in the same cycle 8, 9 or 10 spatial units away (randomly chosen) from the parent in both prey and predators. These higher step values (10 spatial units is the double of maximum dprey and dP) were chosen to reflect that juvenile dispersion distances can far exceed adult movement ranges.
Detection networks
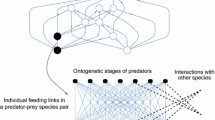
From the spatial distribution of prey, we defined detection networks based on the range within which individuals could observe the behaviour of others (i.e., exploit social cues if present) in each simulation cycle (Fig. 2). In such networks, nodes represent individuals, and edges denote the possibility of mutual observation. If a prey individual became alarmed because it successfully detected a predator, information could spread from this individual to other conspecifics in the network under the following rules. The probability of information acquisition from one node to another is given by wk, where w is the edge weight (corresponding to the probability of information spread from one node to another through the edge between them and specified by the parameter Pisi in the model) and k is the number of steps on the shortest path between the two nodes. Only shortest paths were used to minimise the “travel time” of information between nodes in the network. During simulations, the maximum number of steps between the focal and the observed nodes was set to two and the total number of observed neighbours to ten (i.e., kmax = 2 and ∑(n)max = 5 in each k step). Thus, an individual could receive information from a maximum of ten of its neighbours that were a maximum of two steps away in the detection network. With such restrictions, ISI use did not facilitate the emergence of large aggregations in prey and did not occur far outside the hunting range of predators. If there were more than five nodes at k step to a focal node, we randomly selected five. For any individual, the total probability of receiving information from its neighbours was calculated using the inclusion–exclusion principle (Allenby and Slomson 2010).
Schematic figure of a detection network (a) and segment of an individual ego network embedded within that network (b). Nodes represent individuals and edges denote the possibility of mutual observation. The probability of information acquisition from one node to another is given by w.k, where w is the edge weight and k is the number of steps on the shortest path between the two nodes. For any individual, the total probability of receiving information from neighbours is calculated using the inclusion–exclusion principle. In our model, we used the settings kmax = 2 and ∑nmax = 5 in each k step, so the focal individual (black circle) could receive social information from a maximum of ten neighbours that were a maximum of two steps away in the detection network (orange circles)
Analysis of simulation outputs
All simulations and calculations were performed in R 4.0.4 (R Core Team 2021). Instead of frequentist hypothesis testing, we focused on evaluating the magnitude of differences between simulation runs with different parameter settings (White et al. 2014). We ran the population simulations for 200 cycles (this interval was sufficient to reach equilibrium prey population size in the studied scenarios; see in Fig. 3a) and used the data from the last cycle in all calculations.
Effects of the Pdetect and Pisi model parameters on the prey population. a Temporal fluctuations in prey abundance (means with range) without predators (grey) and under nominal parameter settings (with nominal Pdetect and Pisi – orange, with nominal Pdetect – dark blue, with minimal Pdetect – light blue). When the predator detection probability was set to its minimal value, the prey population died out in a single iteration; in all other cases, the number of iterations was set to 50. b Results of the global sensitivity analysis (SA) depicting the impact of each model parameter on the mean (x-axis) and standard deviation (y-axis) of prey abundance; mean ± SD values for each parameter were calculated from five independent SA runs. Inset shows the model parameters ordered according to their overall influence on the model output
We characterised prey population sizes by calculating the mean, standard deviation, maximum and minimum values in four settings: in the absence of predators, with minimal Pdetect, with nominal Pdetect, and with nominal Pdetect and Pisi parameter values, respectively. All other parameters were set to their initial values; for each model type, simulations were iterated 50 times. When the predator detection probability was set to its minimal value, the prey population died out in a single iteration; prey extinction was not observed in other settings.
We examined how predator abundance affected mortality rate due to predation in prey in the presence of minimal Pdetect, nominal Pdetect, and nominal Pdetect and Pisi parameter values, respectively. All other parameters were set to their initial values. In each setting, simulation runs were iterated 50 times. If the prey population died out before the 200th simulation cycle, the given run was omitted from the dataset (n = 209; only in the ‘minimal predator detection’ setting).
Sensitivity analysis
We used Morris’s “OAT” elementary effects screening method (Morris 1991) with the extension introduced by Campolongo et al. (2007) as a global sensitivity analysis (SA) to rank the model parameters according to their impact on prey population size. We chose this SA because it produces results comparable to the more complex methods (Confalonieri et al. 2010) and is applicable to uncover the mechanisms and patterns produced by individual-based models (Imron et al. 2012; Beaudouin et al. 2015; ten Broeke et al. 2016). The mean of the absolute value of the elementary effect (\({{\mu }^{*}}_{i}\)) provides a measure for the overall influence of each input variable on the model output, whereas the standard deviation of the elementary effect (\({\sigma }_{i})\) indicates possible non-linear effects or interactions among variables (Campolongo et al. 2007; Iooss and Lemaître 2015). We also ranked the model parameters using a global index (GI) (Ciric et al. 2012) calculated as:
For the space-filling sampling strategy proposed by Campolongo et al. (2007), we generated r2 = 1000 Morris trajectories and then retained r1 = 50 with the highest ‘spread’ in the input space to calculate the elementary effect for each model parameter.
Parameter space exploration
We explored a specific part of the parameter space by visualising the combined effect of the parameters Pdetect, Pisi, λP and λreduced on prey population size. Specifically, we investigated the effect of ISI use at low, intermediate and high levels of predator detection probabilities. In each scenario, predator avoidance behaviour had either no cost or incurred moderate fitness cost (i.e., decreased by one third compared to the maximum) and predation pressure was either low (0.025), intermediate (0.05) or high (0.075). In each setting, we used the complete range of parameter values for Pisi (Table 1). Simulations were iterated 30 times. In the low predator detection probability scenario coupled with high predation pressure, the prey population died out in the majority of simulation runs (n = 581); these simulation outputs were omitted from the assembled dataset.
Network characterisation
We generated network data by running the model with λP = 0.075 (i.e., under a high level of predation pressure), while all other parameters were set to their nominal values. We compared emerging detection networks that were generated with the presence of ISI use (Pisi = 0.5) to those that were obtained when Pisi = 0. We calculated the number of components, component size, average ego network and average global efficiency as structural network properties for network characterisation. Simulations were repeated 50 times in each parameter setting. Additionally, we calculated the same characteristics for randomised detection networks as well (Farine 2017; Hobson et al. 2021). These were constructed from both type of the observed detection networks by randomly reshuffling the edges between nodes while also retaining the original degree distributions. Thus, randomised networks represented a hypothetical scenario where interactions are equally likely between any pair of nodes (Croft et al. 2011). While the main purpose here was to explore the global structure of the observed detection networks, the randomised networks helped us to assess whether ISI use could similarly affect the structural properties of networks that were based on this simplifying assumption. The number of components represents the number of connected parts in the detection networks (isolated nodes excluded). We computed component size as the number of components divided by the number of connected nodes; this measure denotes the average number of nodes embedded within components. We calculated the average size of ego networks as the mean number of reachable nodes within two steps in the components. To estimate transmissibility within components, we used the measure ‘global efficiency’ (Latora and Marchiori 2001; Pasquaretta et al. 2014; Romano et al. 2018). Global efficiency for a graph with N vertices is:
where dij is the shortest path length between nodes i and j. The value of this measure ranges from 0 to 1, and represents how fast information may spread from the source to the most peripheral network positions with the least number of connections (Romano et al. 2018). We computed global efficiency for the largest components in the networks. For the calculation of the above network properties, we used the ‘igraph’ and ‘brainGraph’ R packages (Csardi and Nepusz 2006; Watson 2020).
Results
We found that nominal predation pressure coupled with minimal predator detection probability (Pdetect = 0.1) led to small prey population size with high variation among runs compared to the null model when predators were absent and prey population existed at the carrying capacity of the environment (Fig. 3a, Table 2). Nominal predator detection probability (Pdetect = 0.5) increased mean prey population size and stabilised the prey population at higher abundance values, while in the presence of nominal probability of ISI use in prey (Pdetect = 0.5, Pisi = 0.5), prey population size increased further by approx. 53%. The sensitivity analysis also confirmed that Pisi was an influential model input in the constructed model (Fig. 3b). As expected, the parameters driving antipredator behaviour, i.e. the level of predation pressure, the probability of predator detection directly or via conspecifics, and the cost associated with performing antipredator behaviour, were all important and characterised by non-linear effects on prey abundance and/or strong interactions with other parameters. The parameters dprey and dP had considerably less influence on the dispersion of the model output, and were fixed to their nominal values in the subsequent analyses. The mechanism behind the effect of Pisi was that the presence of ISI use could decrease the per capita mortality due to predation across the whole range of the examined predation pressure regime and substantially mitigate the positive relationship between predation-related mortality rate and predator population size (Fig. 4, Fig. S1).
The relationship between per capita mortality due to predation and the number of predators using the same parameter settings as in Fig. 3a (but without the ‘No predators’ group). Trend lines were fitted using second-order polynomial approximation. Simulation results from incomplete runs (i.e., simulation cycles were less than 200) were omitted from the dataset (n = 204; only in the ‘minimal predator detection’ model type)
Consistent with expectations, Pisi affected prey number in all examined Pdetect scenarios in interaction with the effect of cost and predator pressure (Fig. 5). This relationship was positive and non-linear in most cases. When the predation pressure was low, Pisi positively influenced prey abundance to a limited extent, while the effect of the associated cost, especially at lower Pisi values, depended on the value of Pdetect. When the predation pressure was intermediate or high, Pisi exerted a more substantial influence on prey abundance and had the capacity to double the number of prey individuals irrespective of the presence or absence of associated cost (Table S1). Importantly, ISI use could counteract high predation pressure only when Pdetect had a sufficient value (directly dependent on the degree of predation pressure), and did not compensate for low predator detection ability as indicated by the high prevalence of population extinctions in prey when high predation pressure was coupled with low predator detection ability. The presence of associated fitness cost in the high predation pressure settings greatly reduced the magnitude of the effect of ISI use on prey population size, but Pisi could still increase prey population size even at intermediate values if Pdetect > 0.25.
Interactive effects of the probability of ISI use (Pisi), predation pressure (λP), and the presence of fitness cost (associated with the defensive behaviour; λreduced) on prey population size in three Pdetect scenarios. The colour of the boxplots indicates the level of predation pressure (purple: high, blue: intermediate, green: low), while the colour tone is associated with the presence of cost (dark: costly defensive behaviour, light: no cost). Trend lines were fitted using the ‘LOESS’ regression method for smoothing with the default value of span (0.75), presented only for illustration purposes. Simulation results from incomplete runs were omitted from the dataset (n = 581; only in the ‘Pdetect = 0.25’ setting)
The observed detection networks were characterized by high numbers of components that consisted of few connected individuals and small ego networks (Fig. 6, Table S2). The number of connected individuals more than tripled when social information could spread among individuals, while the number of isolates did not change with ISI use. Twice as much components were found in the observed detection networks in the presence of ISI use compared to the setting when it was absent; this effect, however, was not detectable in the randomised counterparts. Mean component size was unaffected by the presence of ISI use in the observed networks, but increased substantially in the randomised ones. Ego network sizes were similarly influenced by ISI use in both network types. Global efficiency within the largest components was high in the absence of ISI use in both observed and randomised networks; however, it was also high in the presence of ISI use in the observed detection networks, indicating efficient information transmission among individuals whenever connected prey was able to detect nearby predators. These attributes of functioning detection networks were unlikely to be the direct consequence of higher prey population size in the presence of ISI use, because the corresponding randomised networks did not show the same degree of structural changes compared to the Pisi = 0 setting.
Four structural network properties (a number of components, b component size, c average size of ego networks, d average global network efficiency) calculated for the observed detection networks (circles) and corresponding randomised networks (diamonds). Predation pressure was set to ‘high’ (i.e., λP = 0.075). Boxplots show the median and interquartile range, whiskers denote values within 1.5-fold of the interquartile range, and dots are individual values. The colour of the boxplots indicates the absence (grey; Pisi = 0) or presence of ISI use (orange; Pisi = 0.5)
Discussion
Social information use has been assumed both to increase individual fitness and to affect population- and community-level processes (Dall et al. 2005; Gil et al. 2018). We expected that such effects could emerge in randomly moving non-grouping prey if behavioural contagion can occur through detection networks, i.e., a dynamic system of temporary observation-based connections between conspecifics. Correlated random walk has successfully been used to describe non-oriented movement trajectory in a number of non-grouping animals, e.g. insects (e.g., Kareiva and Shigesada 1983; McCulloch and Cain 1989; Byers 2001), zooplankton (e.g., Komin et al. 2004; Uttieri et al. 2004, 2008), echinoderms (e.g., Lohmann et al. 2016) or mammals (e.g., Johnson et al. 2008). We found that irrespective of the apparent stochasticity in our model, the sharing of adaptive antipredator behaviour could contribute to population stability and persistence in prey by mitigating predation-related per capita mortality and raising equilibrium population sizes. We also showed that temporary detection networks had structural properties that allowed the efficient spread of adaptive antipredator behaviour among prey under high predation pressure. In group-living animals, information spreads via social connections among individuals and social network positions strongly interact with individual spatial behaviour (Firth and Sheldon 2016; Spiegel et al. 2016; Webber and Vander Wal 2018; Albery et al. 2021), thus movement characteristics and space use are shaping information transmission by affecting social connections. Our findings indicate that non-grouping animals, by being embedded in detection networks based on their perception attributes and spatial locations, can benefit from similar information transmission processes as well. As inadvertent social information use in non-grouping animals is largely understudied (see in Tóth et al. 2020), in the following paragraphs we contrasted our simulation results on non-grouping prey with previous (empirical) findings on group-living species in several instances.
Our results corroborate with previous studies on group-living organisms indicating that social information may act as a stabilising mechanism in systems where predators can exert high pressure on prey populations (Gil et al. 2017, 2018, 2019). While in those models social information directly reduced (following a specific function) the per capita mortality (e.g., Gil et al. 2018), the presented work offers a more mechanistic understanding of how inadvertent social information could propagate through a population of randomly moving individuals. Our findings indicate that predator detection ability had to reach a sufficient level, strongly dependent on the actual level of predation pressure, for ISI use to facilitate prey population persistence. Notably, when this condition was met, ISI use exerted a detectable positive influence on prey population size by relaxing predation pressure even at low probabilities and even if the adaptive antipredator behaviour incurred a fitness cost. Although the depth of our understanding of the detected non-linear relationships and potential thresholds is limited by their coarse-grained variation in these parameters examined here, simulations nonetheless prove that in a substantial part of the parameter space social information use can be expected to raise non-grouping prey population size and facilitate its persistence. These findings may have crucial implications in many theoretical and applied ecological contexts, ranging from the invasive dynamics of predator–prey systems to the efficiency of biological control practices. For instance, the recognition of novel predators by naïve prey has been associated with social information use via different perception modalities in group-living fish (Ferrari et al. 2005; Manassa et al. 2013), and similar utilisation of social cues in social birds has been shown to facilitate the spread of novel aposematic prey (Thorogood et al. 2018; Hämäläinen et al. 2021a, b). Such social information-mediated interactions between prey and predators might be more prevalent in natural ecosystems that include non-grouping species as well, contributing to deviations from the predictions of theoretical models in the dynamics of trophic interactions (Polis et al. 2000). When natural enemies are used as biological control agents for pest management, diffusion of antipredator responses among prey may substantially reduce predation rates rendering these practices less effective and profitable. Besides, it may also mitigate the expected positive impact of the non-consumptive effects of predators (NCEs; Preisser et al. 2007; Sih et al. 2009) such as decreased crop damage due to reduced feeding rate in pests (Beleznai et al. 2017; Tholt et al. 2018). This inflation of NCEs due to information spread can generate discrepancies in the findings of large-scale field studies and laboratory experiments (see in Weissburg et al. 2014), and should be taken into consideration in investigations that aim to evaluate how NCEs may trigger trophic cascades in different ecosystems (Hermann and Landis 2017; Haggerty et al. 2018; Pessarrodona et al. 2019).
Detection networks had distinct structural characteristics when prey experienced high predation pressure and exploited social cues to avoid predators. These networks typically consisted of many components with few connected individuals and small average ego networks, and within these small components, social information could spread with relatively high efficiency. The key to understanding the differences in structural properties of detection networks in the presence and absence of ISI use lies in identifying the process that generates more and smaller components. One plausible explanation is that prey distribution in the simulated landscape could remain more homogeneous due to a decreased susceptibility to predation in the vicinity of predators as the diffusion of social information greatly enhances the probability of predator detection even among a few nearby individuals. While high network efficiency has previously been identified in small animal groups, cognitive abilities and strong social affiliations have usually been involved in explaining this emergent property (Waters and Fewell 2012; Pasquaretta et al. 2014). Our findings indicate that incidental connections among non-grouping animals may generate networks that have similar favourable attributes. In addition to differences in the sizes of connected components, there may be other key differences in how information spreads through detection or sensory networks among group-living (Strandburg-Peshkin et al. 2013; Rosenthal et al. 2015; Davidson et al. 2021) and non-grouping individuals, however. First, behavioural contagion can be complex, and the number of non-alarmed individuals within the detection range influences the likelihood of adopting a specific behaviour (Firth 2020). Previous works on social species have provided mounting evidence for such complex contagion (Hoppitt and Laland 2013; Grüter and Leadbeater 2014; Kendal et al. 2018). Second, imperfect copying might decrease the intensity of behavioural responses with each transmission step, and under a given threshold intensity, social cues exert no response from nearby observers. In this case, individuals’ ability to convey information about predation hazards is related to the extent of behavioural change compared to a baseline level (Chivers and Ferrari 2014). Third, phenotypic heterogeneity among individuals may influence information diffusion if individual traits (e.g., related to hunger, age or developmental stage) or functional traits that transcend species (e.g., similarity in body size that may lead to shared predators) affects the individual capacity to produce social information (Farine et al. 2015).
To describe how ISI use may affect population dynamics in non-grouping prey, we constructed a tentative model with naturalistic predator-to-prey ratios (1:1.03 [when predator detection probabilities was set to minimal]–1:4.23 [with nominal predator detection and ISI use probabilities]; see in Donald and Anderson 2003). Previous observations indicate that predator detection probability, which has been found to play a crucial role in the emergence of social information-mediated effects in our study, can have a value within the upper half of the range examined here (i.e., > 0.5) under relevant conditions (e.g., Tisdale and Fernández-Juricic 2009; Manzur et al. 2018). However, being strongly dependent on the neuronal pathways underlying detection mode and the processing capacity of the brain (Clark and Dukas 2003; Pereira and Moita 2016), it can differ significantly between species and even within the same species as it may also depend on the forager’s state of energy reserves (Clark and Mangel 2000). Therefore, to construct a more realistic model, both species-specific and context-specific information (e.g., movement distances, detection ranges and reproduction rates) for existing predator–prey relationships need to be incorporated, which can be done only at the expense of generality. Model precision may be further enhanced by incorporating additional variables including the functional response of specific predator species (Dunn and Hovel 2020), different non-consumptive effects (other than reduced feeding rate) (Peckarsky et al. 2008), a measure of social cue reliability (Dunlap et al. 2016), social information use in predators (Hämäläinen et al. 2021a, b) or landscape heterogeneity that could alter the space use of individuals (Albery et al. 2021). The effects of different transmission modes can also be tested, for instance, by weighting the probability of information diffusion among conspecifics by the proportion of alarmed and non-alarmed individuals within the detection zone or incorporating heterogeneity among individuals in attributes that affect their propensity to act as social cue producers. Our work, thus, represents a general modelling approach that could be applied to predator–prey systems in which populations are demographically decoupled and non-grouping prey may mitigate predation hazards through the exploitation of incidentally produced social information.
Data availability
Data files supporting the results and R script for model construction and simulated data are archived and available at Figshare (https://figshare.com/s/34fc714342dab9123193). Upon reasonable requests, R codes for the model functions are also available from the corresponding author.
References
Albery GF, Morris A, Morris S, Pemberton JM, Clutton-Brock TH, Nussey DH, Firth JA (2021) Multiple spatial behaviours govern social network positions in a wild ungulate. Ecol Lett 24:676–686
Allenby RBJT, Slomson A (2010) How to count: an introduction to combinatorics, discrete mathematics and its applications, 2nd edn. CRC Press, Boca Raton, pp 51–60
Beaudouin R, Goussen B, Piccini B, Augustine S, Devillers J, Brion F, Péry AR (2015) An individual-based model of zebrafish population dynamics accounting for energy dynamics. PLoS One 10:e0125841
Beleznai O, Dreyer J, Tóth Z, Samu F (2017) Natural enemies partially compensate for warming induced excess herbivory in an organic growth system. Sci Rep 7:7266
Benhamou S (2006) Detecting an orientation component in animal paths when the preferred direction is individual-dependent. Ecology 87:518–528
Bonnie KE, Earley RL (2007) Expanding the scope for social information use. Anim Behav 74:171–181
Boujja-Miljour H, Leighton PA, Beauchamp G (2017) Spread of false alarms in foraging flocks of house sparrows. Ethology 123:526–531
Brown GE, Godin J-GJ, Pedersen J (1999) Fin-flicking behaviour: a visual antipredator alarm signal in a characin fish, Hemigrammus erythrozonus. Anim Behav 58:469–475
Byers JA (2001) Correlated random walk equations of animal dispersal resolved by simulation. Ecology 82:1680–1690
Campolongo F, Cariboni J, Saltelli A (2007) An effective screening design for sensitivity analysis of large models. Environ Model Softw 22:1509–1518
Červený J, Begall S, Koubek P, Nováková P, Burda H (2011) Directional preference may enhance hunting accuracy in foraging foxes. Biol Lett 7:355–357
Chivers DP, Ferrari MC (2014) Social learning of predators by tadpoles: does food restriction alter the efficacy of tutors as information sources? Anim Behav 89:93–97
Ciric C, Ciffroy P, Charles S (2012) Use of sensitivity analysis to identify influential and non-influential parameters within an aquatic ecosystem model. Ecol Modell 246:119–130
Clark CW, Dukas R (2003) The behavioral ecology of a cognitive constraint: limited attention. Behav Ecol 14:151–156
Clark CW, Mangel M (2000) Dynamic state variable models in ecology. Oxford University Press, New York
Codling EA, Plank MJ, Benhamou S (2008) Random walk models in biology. J R Soc Interface 5:813–834
Coleman SW (2008) Mourning dove (Zenaida macroura) wing-whistles may contain threat-related information for con-and hetero-specifics. Sci Nat 95:981
Confalonieri R, Bellocchi G, Bregaglio S, Donatelli M, Acutis M (2010) Comparison of sensitivity analysis techniques: a case study with the rice model WARM. Ecol Modell 221:1897–1906
Coolen I, Dangles O, Casas J (2005) Social learning in noncolonial insects? Curr Biol 15:1931–1935
Crane AL, Demers EE, Feyten LE, Ramnarine IW, Brown GE (2022) Exploratory decisions of Trinidadian guppies when uncertain about predation risk. Anim Cogn 25:581–587
Croft DP, Madden JR, Franks DW, James R (2011) Hypothesis testing in animal social networks. Trend Ecol Evol 26:502–507
Cruz A, Heinemans M, Marquez C, Moita MA (2020) Freezing displayed by others is a learned cue of danger resulting from co-experiencing own freezing and shock. Curr Biol 30:1128–1135
Csardi G, Nepusz T (2006) The igraph software package for complex network research. Int J Complex Syst 1695:1–9
Dall SR, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW (2005) Information and its use by animals in evolutionary ecology. Trends Ecol Evol 20:187–193
Dall SR, Johnstone RA (2002) Managing uncertainty: information and insurance under the risk of starvation. Phil Trans R Soc B 357:1519–1526
Danchin E, Giraldeau L-A, Valone TJ, Wagner RH (2004) Public information: from nosy neighbors to cultural evolution. Science 305:487–491
Davidson JD, Sosna MMG, Twomey CR, Sridhar VH, Leblanc SP, Couzin ID (2021) Collective detection based on visual information in animal groups. J R Soc Interface 18:20210142
Donald DB, Anderson RS (2003) Resistance of the prey-to-predator ratio to environmental gradients and to biomanipulations. Ecology 84:2387–2394
Duboscq J, Romano V, MacIntosh A, Sueur C (2016) Social information transmission in animals: lessons from studies of diffusion. Front Psychol 7:1147
Dunlap AS, Nielsen ME, Dornhaus A, Papaj DR (2016) Foraging bumble bees weigh the reliability of personal and social information. Curr Biol 26:1195–1199
Dunn RP, Hovel KA (2020) Predator type influences the frequency of functional responses to prey in marine habitats. Biol Lett 16:20190758
Farine DR (2017) A guide to null models for animal social network analysis. Methods Ecol Evol 8:1309–1320
Farine DR, Montiglio PO, Spiegel O (2015) From individuals to groups and back: the evolutionary implications of group phenotypic composition. Trends Ecol Evol 30:609–621
Ferrari MC, Trowell JJ, Brown GE, Chivers DP (2005) The role of learning in the development of threat-sensitive predator avoidance by fathead minnows. Anim Behav 70:777–784
Firth JA (2020) Considering complexity: animal social networks and behavioural contagions. Trends Ecol Evol 35:100–104
Firth JA, Sheldon BC (2016) Social carry-over effects underpin trans-seasonally linked structure in a wild bird population. Ecol Lett 19:1324–1332
Galef BG Jr, Giraldeau L-A (2001) Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim Behav 61:3–15
Gil MA, Baskett ML, Schreiber SJ (2019) Social information drives ecological outcomes among competing species. Ecology 100:e02835
Gil MA, Emberts Z, Jones H, St Mary CM (2017) Social information on fear and food drives animal grouping and fitness. Am Nat 189:227–241
Gil MA, Hein AM (2017) Social interactions among grazing reef fish drive material flux in a coral reef ecosystem. P Natl Acad Sci USA 114:4703–4708
Gil MA, Hein AM, Spiegel O, Baskett ML, Sih A (2018) Social information links individual behavior to population and community dynamics. Trends Ecol Evol 33:535–548
Goodale E, Beauchamp G, Magrath RD, Nieh JC, Ruxton GD (2010) Interspecific information transfer influences animal community structure. Trends Ecol Evol 25:354–361
Goodale E, Beauchamp G, Ruxton GD (2017) Mixed-species groups of animals: behavior, community structure, and conservation. Academic Press, London
Greggor AL, Thornton A, Clayton NS (2017) Harnessing learning biases is essential for applying social learning in conservation. Behav Ecol Sociobiol 71:16
Grüter C, Leadbeater E (2014) Insights from insects about adaptive social information use. Trends Ecol Evol 29:177–184
Haggerty MB, Anderson TW, Long JD (2018) Fish predators reduce kelp frond loss via a trait-mediated trophic cascade. Ecology 99:1574–1583
Hämäläinen L, Hoppitt W, Rowland HM, Mappes J, Fulford AJ, Sosa S, Thorogood R (2021a) Social transmission in the wild can reduce predation pressure on novel prey signals. Nat Commun 12:3978
Hämäläinen L, Rowland HM, Mappes J, Thorogood R (2021b) Social information use by predators: expanding the information ecology of prey defences. Oikos (published online. https://doi.org/10.1111/oik.08743)
Hermann SL, Landis DA (2017) Scaling up our understanding of non-consumptive effects in insect systems. Curr Opin Insect Sci 20:54–60
Hingee M, Magrath RD (2009) Flights of fear: a mechanical wing whistle sounds the alarm in a flocking bird. Proc R Soc Lond B 276:4173–4179
Hobson EA, Silk MJ, Fefferman NH, Larremore DB, Rombach P, Shai S, Pinter-Wollman N (2021) A guide to choosing and implementing reference models for social network analysis. Biol Rev 96:2716–2734
Hoppitt W, Laland KN (2013) Social learning: an introduction to mechanisms, methods, and models. Princeton University Press, Princeton
Imron MA, Gergs A, Berger U (2012) Structure and sensitivity analysis of individual-based predator–prey models. Reliab Eng Syst Safe 107:71–81
Iooss B, Lemaître P (2015) A review on global sensitivity analysis methods. In: Dellino G, Meloni C (eds) Uncertainty management in simulation-optimization of complex systems. Springer, Boston, pp 101–122
Johnson DS, London JM, Lea MA, Durban JW (2008) Continuous-time correlated random walk model for animal telemetry data. Ecology 89:1208–1215
Kareiva PM, Shigesada N (1983) Analyzing insect movement as a correlated random walk. Oecologia 56:234–238
Kendal RL, Boogert NJ, Rendell L, Laland KN, Webster M, Jones PL (2018) Social learning strategies: bridge-building between fields. Trends Cogn Sci 22:651–665
King AJ, Cowlishaw G (2007) When to use social information: the advantage of large group size in individual decision making. Biol Lett 3:137–139
Komin N, Erdmann U, Schimansky-Geier L (2004) Random walk theory applied to Daphnia motion. Fluct Noise Lett 4:L151–L159
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, New York
Laland KN (1992) A theoretical investigation of the role of social transmission in evolution. Ethol Sociobiol 13:87–113
Latora V, Marchiori M (2001) Efficient behavior of small-world networks. Phys Rev Lett 87:198701
Lima SL (1990) The influence of models on the interpretation of vigilance. In: Bekoff M, Jamieson D (eds) Interpretation and explanation in the study of animal behavior: Explanation, evolution and adaptation, vol 2. Westview Press, Boulder, pp 246–267
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lohmann AC, Evangelista D, Waldrop LD, Mah CL, Hedrick TL (2016) Covering ground: movement patterns and random walk behavior in Aquilonastra anomala sea stars. Biol Bull 231:130–141
Manassa RP, McCormick MI, Chivers DP, Ferrari MCO (2013) Social learning of predators in the dark: understanding the role of visual, chemical and mechanical information. Proc R Soc B 280:20130720
Manzur T, Gonzalez-Mendez A, Broitman BR (2018) Scales of predator detection behavior and escape in Fissurella limbata: a field and laboratory assessment. Mar Ecol 39:e12492
Martín J, Luque-Larena JJ, López P (2006) Collective detection in escape responses of temporary groups of Iberian green frogs. Behav Ecol 17:222–226
McCulloch CE, Cain ML (1989) Analyzing discrete movement data as a correlated random walk. Ecology 70:383–388
Michálek O, Petráková L, Pekár S (2017) Capture efficiency and trophic adaptations of a specialist and generalist predator: a comparison. Ecol Evol 7:2756–2766
Morris MD (1991) Factorial sampling plans for preliminary computational experiments. Technometrics 33:161–174
O’Mara MT, Dechmann DK, Page RA (2014) Frugivorous bats evaluate the quality of social information when choosing novel foods. Behav Ecol 25:1233–1239
Parejo D, Avilés JM (2016) Social information use by competitors: resolving the enigma of species coexistence in animals? Ecosphere 7:e01295
Pasquaretta C, Levé M, Claidiere N et al (2014) Social networks in primates: smart and tolerant species have more efficient networks. Sci Rep 4:7600
Pays O, Beauchamp G, Carter AJ, Goldizen AW (2013) Foraging in groups allows collective predator detection in a mammal species without alarm calls. Behav Ecol 24:1229–1236
Peckarsky BL, Abrams PA, Bolnick DI et al (2008) Revisiting the classics: considering nonconsumptive effects in textbook examples of predator–prey interactions. Ecology 89:2416–2425
Pereira AG, Moita MA (2016) Is there anybody out there? Neural circuits of threat detection in vertebrates. Curr Opin Neurobiol 41:179–187
Pessarrodona A, Boada J, Pagès JF, Arthur R, Alcoverro T (2019) Consumptive and non-consumptive effects of predators vary with the ontogeny of their prey. Ecology 100:e02649
Polis GA, Sears AL, Huxel GR, Strong DR, Maron J (2000) When is a trophic cascade a trophic cascade? Trends Ecol Evol 15:473–475
Preisser EL, Orrock JL, Schmitz OJ (2007) Predator hunting mode and habitat domain alter nonconsumptive effects in predator–prey interactions. Ecology 88:2744–2751
R Core Team (2021) R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. http://www.R-project.org
Reynolds AM (2014) Towards a mechanistic framework that explains correlated random walk behaviour: correlated random walkers can optimize their fitness when foraging under the risk of predation. Ecol Complex 19:18–22
Romano V, Shen M, Pansanel J, MacIntosh AJ, Sueur C (2018) Social transmission in networks: global efficiency peaks with intermediate levels of modularity. Behav Ecol Sociobiol 72:154
Rosenthal SB, Twomey CR, Hartnett AT, Wu HS, Couzin ID (2015) Revealing the hidden networks of interaction in mobile animal groups allows prediction of complex behavioral contagion. P Natl Acad Sci USA 112:4690–4695
Sih A, Hanser SF, McHugh KA (2009) Social network theory: new insights and issues for behavioral ecologists. Behav Ecol Sociobiol 63:975–988
Spiegel O, Leu ST, Sih A, Bull CM (2016) Socially interacting or indifferent neighbours? Randomization of movement paths to tease apart social preference and spatial constraints. Methods Ecol Evol 7:971–979
Strandburg-Peshkin A, Twomey CR, Bode NW et al (2013) Visual sensory networks and effective information transfer in animal groups. Curr Biol 23:R709–R711
ten Broeke G, van Voorn G, Ligtenberg A (2016) Which sensitivity analysis method should I use for my agent-based model? J Artif Soc S 19:5
Tholt G, Kis A, Medzihradszky A, Szita É, Tóth Z, Havelda Z, Samu F (2018) Could vectors’ fear of predators reduce the spread of plant diseases? Sci Rep 8:8705
Thorogood R, Kokko H, Mappes J (2018) Social transmission of avoidance among predators facilitates the spread of novel prey. Nat Ecol Evol 2:254–261
Tisdale V, Fernández-Juricic E (2009) Vigilance and predator detection vary between avian species with different visual acuity and coverage. Behav Ecol 20:936–945
Toland BR (1987) The effect of vegetative cover on foraging strategies, hunting success, and nesting distribution of American kestrels in central Missouri. J Raptor Res 21:14–20
Tóth Z (2021) The hidden effect of inadvertent social information use on fluctuating predator–prey dynamics. Evol Ecol 35:101–114
Tóth Z, Jaloveczki B, Tarján G (2020) Diffusion of social information in non-grouping animals. Front Ecol Evol 8:586058
Uttieri M, Mazzocchi MG, Nihongi A, D’Alcalà MR, Strickler JR, Zambianchi E (2004) Lagrangian description of zooplankton swimming trajectories. J Plankton Res 26:99–105
Uttieri M, Paffenhöfer GA, Mazzocchi MG (2008) Prey capture in Clausocalanus furcatus (Copepoda: Calanoida). The role of swimming behaviour. Mar Biol 153:925–935
Ward A, Webster M (2016) Sociality: the behaviour of group-living animals. Springer International Publishing, New York
Waters JS, Fewell JH (2012) Information processing in social insect networks. PLoS ONE 7:e40337
Watson CG (2020) brainGraph: graph theory analysis of brain MRI data. R package version 3.0.0. https://CRAN.R-project.org/package=brainGraph
Webber QM, Vander Wal E (2018) An evolutionary framework outlining the integration of individual social and spatial ecology. J Anim Ecol 87:113–127
Weissburg M, Smee DL, Ferner MC (2014) The sensory ecology of nonconsumptive predator effects. Am Nat 184:141–157
White JW, Rassweiler A, Samhouri JF, Stier AC, White C (2014) Ecologists should not use statistical significance tests to interpret simulation model results. Oikos 123:385–388
Whitehead H, Laland KN, Rendell L, Thorogood R, Whiten A (2019) The reach of gene–culture coevolution in animals. Nat Commun 10:2405
Acknowledgements
We are indebted to Béla Keresztfalvi for providing access to the institutional IT resources, with which we were able to reduce the computational time of the performed simulations radically. We are also thankful to the reviewers for their constructive comments.
Funding
Open access funding provided by ELKH Centre for Agricultural Research. ZT was financially supported by the Prémium Postdoctoral Research Programme of the Hungarian Academy of Sciences (MTA, PREMIUM-2018–198), by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (MTA, BO/00634/21/8) and by the New National Excellence Program of the Ministry for Innovation and Technology (ITM, ÚNKP-21–5-DE-478) from the source of the National Research, Development and Innovation Fund. GC was financially supported by the Young Researcher Programme of the Hungarian Academy of Sciences (MTA, Mv-41/2020).
Author information
Authors and Affiliations
Contributions
ZT and CG conceived and designed the study. ZT constructed the model, performed the simulations, analysed the model output, wrote the initial manuscript, and revised and edited the subsequent versions. CG contributed substantially to the text and revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by J. Lindström
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tóth, Z., Kőmüves, G. Social information-mediated population dynamics in non-grouping prey. Behav Ecol Sociobiol 76, 110 (2022). https://doi.org/10.1007/s00265-022-03215-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03215-4