Abstract
Research on several social fishes has revealed that shoals constituted by familiar individuals behave remarkably differently compared to shoals formed by unfamiliar individuals. However, whether these behavioural changes may arise also in shoals composed by a mixture of familiar and unfamiliar individuals, a situation that may commonly occur in nature, is not clear. Here, we observed the behaviour of Mediterranean killifish (Aphanius fasciatus) shoals that were composed by both familiar and unfamiliar individuals (i.e. individuals were familiar to each other in pairs) and compared it with shoals entirely made by either unfamiliar or familiar individuals. Shoals formed by familiar individuals took longer to emerge from a refuge and swam more cohesively compared to shoals formed by unfamiliar fish. Shoals formed by a mixture of familiar and unfamiliar individuals behaved as shoals formed by unfamiliar individuals. Moreover, mixed shoals did not segregate in pairs according to their familiarity. This study suggests that mixed shoals do not show the behavioural effects of familiarity.
Significance statement
Laboratory studies have compared the behaviour of shoals formed by familiar fish versus shoals formed by unfamiliar fish, finding notable advantages in the former ones, such as improved antipredator and foraging behaviour. However, comparing these two opposite shoal types may not provide information on the natural situation, because in nature, shoals often change composition. We investigated how shoals formed by a mixture of familiar and unfamiliar fish behaved. We analysed shoals’ preference for open environment versus covers and shoals’ swimming cohesion. Results showed that shoals formed by both familiar and unfamiliar individuals mostly behave like shoals entirely formed by unfamiliar individuals. This suggests that the advantages of social groups formed by familiar fish might be hardly seen in nature for species in which shoal composition changes frequently.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Being familiar with social companions has profound effects on animal behaviour (Kareem and Barnard 1982; Sackett and Fredrickson 1987; Wilkinson et al. 2010; Gutmann et al. 2015), in particular for teleost fish (reviewed in Ward and Hart 2003). A large number of studies have compared the behaviour of groups formed by familiar individuals versus groups of unfamiliar individuals. Familiar individuals behave differently from unfamiliar fish by manifesting marked changes in personality traits (Bhat and Magurran 2006; Galhardo et al. 2012), in school cohesiveness (Chivers et al. 1995), in following behaviour (Lucon-Xiccato et al. 2019), in aggressiveness (Wechkin 1975), and in competitive interactions (Utne-Palm and Hart 2000). These behavioural changes have been linked to benefits in terms of foraging (Griffiths et al. 2003; Hart et al. 2014), growth rate (Seppä et al. 2001), and survival under predation risk (Nadler et al. 2021; Chivers et al. 1995; Griffiths et al. 2003, 2004), which may explain why many fish species show an active and strong preference for associating with familiar individuals (Griffiths and Magurran 1999; Frommen and Bakker 2004; Frommen et al. 2007; Gómez-Laplaza and Fuente 2007).
However, in fish species showing frequent fission–fusion events, natural shoals are often composed by a mix of familiar and unfamiliar individuals. For example, a capture-mark-recapture study on wild guppies, Poecilia reticulata, has found that each individual in the shoal was previously associated with on average 15% current shoal mates, and that pairwise associations occurred scarcely (Croft et al. 2004). Whether the behavioural changes due to familiarity (e.g. Chivers et al. 1995; Bhat and Magurran 2006) emerge in shoals formed by both familiar and unfamiliar fish remains unclear. Here, we addressed this issue in the Mediterranean killifish, Aphanius fasciatus, by comparing the behaviour of shoals formed by four familiar fish, shoals formed by four unfamiliar fish, and mixed shoals formed by two pairs of fish that were familiar within pair but unfamiliar between pairs.
In our experiment, we exposed the experimental shoals to a novel environment and we recorded three behavioural traits: the initial latency to leave a refuge, the time spent in the refuge, and the shoal cohesion. Early studies have shown that these and similar behavioural measures changed in response to social familiarity (Chivers et al. 1995; Bhat and Magurran 2006; Lucon-Xiccato et al. 2017). We predicted three possible outcomes for our experiment. In mixed shoals, the two pairs of familiar individuals might behave independently, resulting in two separate shoals or in a shoal behaviour that is intermediate between that of shoals formed by familiar and unfamiliar fish (e.g. Cote et al. 2011). Alternately, the mixed shoals might behave as a shoal formed by either familiar or unfamiliar fish (e.g. Brown and Irving 2014; Lucon-Xiccato and Griggio 2017).
Materials and methods
Study species
The Mediterranean killifish is endemic of the central and eastern coastal zone of the Mediterranean Sea (Whitehead et al. 1986), where it mostly inhabits brackish water habitats, such as lagoons or costal ponds. Genetic studies indicates that populations are relatively isolated, which suggests low individual movement and dispersal (e.g. Maltagliati 1998, 1999). In our sampling site, the Venice Lagoon, Mediterranean killifish are usually found in small, shallow canals rich of vegetation. In this habitat, they form shoals of variable size, ranging from a handful of individuals to more than 50 individuals. Early studies in the laboratory have reported several social behaviours in this species, including tendency to form shoals (Lucon-Xiccato and Griggio 2017; Lucon-Xiccato et al. 2017), selective association with conspecifics (Cattelan and Griggio 2020), following behaviour (Lucon-Xiccato et al. 2019), fission and fusion events in the shoals (Lucon-Xiccato et al. 2019), and development of social familiarity (Lucon-Xiccato et al. 2017, 2019).
Experimental fish
We obtained the experimental fish from the canals of the Venice Lagoon (Italy; 45° 14′ 24.0" N 12° 17′ 15.1" E). The 16th October 2016, we collected approximately 200 female Mediterranean killifish using a seine net. We placed the fish into aerated tanks and transported them to the laboratory at Umberto D’Ancona Hydrobiological station (Chioggia, Italy). We used females because they show greater tendency to display social interactions compared to males (Cattelan and Griggio 2020). In the laboratory, we kept the fish in four 60 × 40 × 35 cm glass tanks. To minimise the impact of the study on the fish, conditions in the maintenance tanks were as similar as possible to natural conditions (Jones et al. 2021). We provided the tanks with constant seawater flow from the lagoon (approximately 10 L/h), ensuring natural levels of salinity, temperature (average 15 °C), and other chemical and biological parameters in the water. Illumination was provided with neon lamps placed on the ceiling and set according to the natural photoperiod of the area (11 h of light per day). The bottom of the tanks was entirely covered by 5 cm of fine sand (approximately 1 mm grain diameter) collected from the sampling site. Approximately 50% percent of the bottom was also covered by clumps of algae (Ulva lactuca) collected every few days in the lagoon. We provided each maintenance tank with an air stone that, along with the continuous flow of water, ensured water oxygenation. The fish could feed on the organisms brought inside the tanks from waterflow from the lagoon; in addition, we daily provided fresh mussels as food. After 14 days of acclimatisation to the laboratory conditions, we started the experimental procedures.
Familiarisation treatment
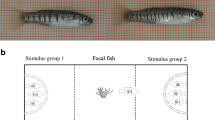
A diagram of the experimental design is showed in Fig. 1a. To obtain fish that were familiar, we allowed 49 groups of four fish to familiarise for 7 days (196 fish overall). We housed each group in a 35 × 35 × 35 cm glass aquarium. Each fish of the group was collected from a different maintenance tank. Care was taken to select fish without signs of distress or disease. Conditions in the familiarisation aquaria were as described for the maintenance tanks, including the presence of flowing seawater, sand bottom, algae and air stones. Food provided as described for the maintenance tanks. Prior studies in this species have shown that this treatment causes the development of familiarity between individuals (Lucon-Xiccato et al. 2017, 2019).
Experimental shoals
At the end of the familiarisation treatment, we composed 49 experimental shoals to be assayed in the behavioural test. The experimental shoals were composed by 4 killifish, in line with the number of individuals (2–6) used in most studies on fish familiarity (Griffiths and Magurran 1999; Bhat and Magurran 2006; Davis et al. 2017; Cattelan et al. 2019). The use of a relatively small shoal allowed to obtain a relatively simple system to test our main hypothesis. Furthermore, the use of small shoals was in line with the ethical requirement of minimising the number of wild subjects used in the study. Each subject was used in a single experimental shoal and therefore tested only once.
We aimed to compare shoals with three compositions (Fig. 1): shoals formed by four familiar individuals, shoals formed by four unfamiliar individuals, and mixed shoals formed by both familiar and unfamiliar individuals. For the first shoal composition (familiar fish), we used four fish that underwent the familiarisation treatment in the same tank (N = 15 shoals). For the second shoal composition (unfamiliar fish), we pooled together four fish that underwent the familiarisation treatment in four different tanks (N = 18 shoals). Last, we composed mixed shoals (N = 16) by pooling two pairs of fish that underwent the familiarisation treatment in two different tanks. As a result, in the mixed shoals, the two individuals of each pair were familiar with each other but were unfamiliar with the individuals of the other pair. We allocated the fish to the different shoals randomly. One experimenter arranged each shoal in a plastic jar (1 L) and a second experimenter (blind with respect of shoal composition) immediately released it into the apparatus for the behavioural testing.
Behavioural testing
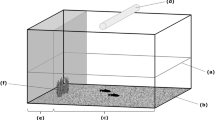
The test apparatus was a 100 × 70 × 50 cm tank filled with 15 cm of seawater (Fig. 1b). The walls of the tank were made of dark grey plastic. The bottom of the apparatus was covered with light-grey gravel bought in a local shop. To prevent external disturbance, dark-green curtains surrounded the apparatus. An 18-W fluorescent lamp was placed above the apparatus to provide illumination. One side of the apparatus (25% surface) was shielded from the light by a plastic panel. This shadow area was provided with green synthetic algae and consisted of a refuge for the fish. The apparatus was therefore divided in a sheltered refuge zone and an open environment. For the behavioural testing, the experimenter placed the fish shoal in the refuge area of the apparatus by gently emptying the jar. After that, we left the experimental room and recorded the behaviour of the subjects for 10 min using a camera placed on the ceiling.
Analyses of the video recordings
The video recordings were coded with a numerical ID. Because the composition of the shoal could not be inferred visually (i.e. all the subjects showed similar phenotypes), scoring of the recordings was blind with respect to the shoal composition. In the analysis of the recordings, we focused on three features of shoal behaviours: latency to emerge from the refuge, time spent in the refuge, and shoal cohesion. The latency to emerge from the refuge zone (s) of each subject was calculated from the moment in which all the fish of a shoal were inserted in the apparatus. We used a value of 600 s if the subject did not leave the refuge for the whole experiment. Regarding the second variable (i.e. the time spent in the refuge zone), we calculated it as a proportion, considering the testing time after the fish left the refuge for the first time, with the formula: (time spent in the refuge—latency to emerge) / (total testing time—latency to emerge). This strategy allowed to decrease the methodological dependency between the time in the refuge variable and the latency to emerge. When computing the proportion of time spent in the refuge, we excluded two fish that did not exit the refuge because the calculation was not possible (i.e. the divisor in the formula was zero); the behaviour of these two subjects was described by the variable ‘latency to emerge’. All the remaining fish exited at least once from the refuge zone and then returned in the refuge zone at least once during the remaining period of the test.
To measure shoal cohesion, we superimposed a 10 × 8 grid to the monitor in correspondence of the open field. Each square corresponded to a 4 × 5 cm sector in the testing apparatus, which roughly corresponded to one fish body length. We used a scan sampling method to sample the position of each subject every two seconds of the experiment (i.e. 300 observations per each shoal) using the position of its head as a reference. We assigned an index of shoal cohesion (Lucon-Xiccato et al. 2017) as follows:
-
value ‘1’ when only one fish could be inscribed into a 3 × 3 squares area;
-
value ‘2’ when only two fish could be inscribed into a 3 × 3 squares area;
-
value ‘3’ when only three fish could be inscribed into a 3 × 3 squares area;
-
value ‘4’ when all the fish could be inscribed into a 3 × 3 squares area.
Therefore, observations in which shoals had high cohesion were assigned an index with greater value. The shoaling index could not be calculated for the observations in which all the four fish were in the refuge.
Statistical analysis
Statistical analysis was performed in R, version 3.2.2 (R Development Core Team 2015). Statistical tests were two-tailed and the significance threshold was α = 0.05. Preliminary plotting suggested covariation between latency to emerge and proportion of time spent in the refuge. Since the testing time was constant among shoals, fish that exhibited smaller latency to emerge had more time to habituate to the apparatus and habituation might affect in turn the proportion of time spent in the refuge. Therefore, it is not clear whether the covariation between these two variables was at least in part driven by our experimental protocol. To handle this issue, we analysed the two variables with a two-steps approach: first, we ran separate models for each variable; then, we ran a third model on a combination of the two variables obtained with principal component analysis (PCA) computed with the ‘prcom’ R function. All these models were linear mixed-effects models (LMMs) fitted using the ‘lme’ function of the ‘nlme’ R package (Pinheiro et al. 2017). The dependent variable was the value of each individual fish of either the latency to emerge from the refuge, the proportion of time in the refuge, or the principal component obtained with the PCA. Shoal ID was fitted as random effect to account for experimental shoals’ replication. The models were fitted with the shoal composition (familiar fish, unfamiliar fish, or mixed) as fixed effect. For the latency to emerge, we conducted log transformation before the analysis because of a right-skewed distribution. Tukey post hoc test computed with the ‘glht’ function of the ‘multcomp’ R package was used to further analyse significant effects of shoal composition. The post hoc test was set to perform all possible pairwise comparisons between shoals with different compositions: familiar versus unfamiliar, mixed versus familiar, and mixed versus unfamiliar.
The shoal cohesion data consisted of the number of observations assigned to each of the four indexes. Therefore, we handled this variable with a generalised linear mixed-effects model (GLMM; ‘glmer’ function of the ‘lme4’ R package; Bates et al. 2014) with binomial error distribution (i.e. without data transformation). As a dependent variable, we used the matrix formed by the number of observations for each index and the total number of valid observations (i.e. observations in which all the fish were in the open environment). For instance, if a shoal is observed 10 times in the open arena and in 7 of these observations, all the fish are in the same 3 × 3 squares area (index value = 4) the data of this shoal for index ‘4’ would be: 7, 10. Expressing the dependent variable as a matrix allowed us to standardise the data according to the number of valid observations of each shoal, controlling for binomial sampling error. Then, we fitted shoal composition and index as fixed effects and shoal ID as random effect in the model to account for sample correlation (i.e. repeated measures). To understand the significant interaction in the model, we ran a set of separate post hoc models, each model fitting only the data relative to a pair of shoal types (familiar versus unfamiliar, familiar versus mixed, or unfamiliar versus mixed).
Results
Latency to emerge
There was a significant effect of shoal composition on latency to emerge from the refuge (F2,46 = 5.299, P = 0.009; Fig. 2). The Tuckey post hoc test indicated that shoals formed by familiar fish emerged from the refuge later compared to shoals formed by unfamiliar fish (P = 0.005) and compared to mixed shoals (P = 0.036). The latency to emerge from the refuge did not differ between mixed shoals and shoals formed by unfamiliar fish (P = 0.831).
Variation in (a) latency to emerge from the refuge zone and (b) proportion of time spent in the refuge zone in relation to the composition of the shoal. In the boxplots, edges represent the upper and lower quartiles, internal lines represent the median, whiskers represent maximum and minimum, and points represent outliers
Time spent in the refuge
The models on the proportion of time spent in the refuge did not reveal a significant effect of shoal composition (F2,46 = 5.299, P = 0.305; Fig. 2b).
PCA of latency to emerge and time spent in the refuge
The PCA conducted on the latency to emerge from the refuge and the time spent in the refuge produced two principal components. The first component (PC1) explained 94.79% of the variance of the two variables. A model conducted on PC1 revealed a significant effect of shoal composition (F2,144 = 5.504, P = 0.007). The Tuckey post hoc test indicated that shoals formed by familiar fish differed in term of PC1 compared to shoals formed by unfamiliar fish (P = 0.005) and compared to mixed shoals (P = 0.028). The PC1 did not differ between mixed shoals and shoals formed by unfamiliar fish (P = 0.852).
Shoal cohesion
The model on the occurrence of shoal cohesion indexes found a significant main effect of index value (Χ23 = 584.401, P < 0.001) and a significant interaction between index value and shoal composition (Χ26 = 192.905, P < 0.001; Fig. 3), but not a significant main effect of shoal composition (Χ22 = 0.267, P = 0.875). Post hoc models revealed that shoals formed by familiar fish showed less often the index value ‘1’, which indicated low cohesion, and more often the index value ‘4’, which indicated high cohesion, compared to shoals formed by unfamiliar fish (Χ23 = 128.833, P < 0.001) and compared to mixed shoals (Χ23 = 162.845, P < 0.001). The frequency of the cohesion indexes did not significantly differ in the comparison between mixed shoals and shoals formed by unfamiliar fish (Χ23 = 3.087, P = 0.378).
Shoal cohesion in relation to the composition of the shoal. Increasing values of the indexes indicated increasing shoal cohesion. In the boxplots, edges represent the upper and lower quartiles, internal lines represent the median, whiskers represent maximum and minimum, and points represent outliers
Discussion
The comparison between shoals formed by familiar and unfamiliar fish revealed effects of familiarisation for two of the three behavioural measures: the latency to emerge from the refuge and the cohesion of shoals in the open environment. Shoals formed by familiar fish took longer to emerge from the refuge and were more likely to show high levels of cohesion compared to shoals formed by unfamiliar fish. Critically, both these familiarisation effects were absent in mixed shoals formed by both familiar and unfamiliar fish. For a third behavioural variable, the time spent in the refuge after emerging in the open environment for the first time, we failed to detect the same effect. However, graphical inspection (Fig. 2b) and a model conducted after a PCA with the latency variable suggested that the time spent in the refuge might follow the same pattern of results described for the other two variables, with the shoals of familiar individuals differing from both the shoals formed by unfamiliar fish and the mixed shoals.
Our findings suggests that Mediterranean killifish shoals formed by two pairs of individuals that were familiar within pair but not between pairs resembled the behaviours of shoals formed by unfamiliar fish. It remains to understand the cause of this effect. It could not be attributed to behavioural segregation of the two pairs of familiar fish within the shoal, because there was no significant difference in the shoal cohesion index 2 (i.e. observations of fish pairs swimming closely) among shoals with different composition. A second potential interpretation would be that the effect was driven by aggressive interactions in the mixed shoals. However, results suggest that this explanation is unlikely for two reasons. First, there was no segregation in the two pairs of the mixed shoals, as expected in case of elevated aggressive behaviour. Second, there was no difference between the mixed shoals and the unfamiliar shoals, and in the latter type of shoals, aggression was expected to be higher. Therefore, the most conservative interpretation is that the familiar pair of fish adjusted their behaviour to that of the unfamiliar shoal mates, resulting in the entire shoal behaving as a shoal formed by unfamiliar fish. Similar cases in which fish adjust their behaviour to that of the shoal mates have been reported in relation to factors such as personality (Magnhagen 2012; Brown and Irving 2014) and sex of the individuals (Lucon-Xiccato et al. 2017), and have been commonly observed in collective movements during foraging (Day et al. 2001; Webster and Hart 2006; Webster and Laland 2012).
Our findings might help to estimate the occurrence of familiarity effects and the associated benefits in nature. Familiarity effects have been usually demonstrated with laboratory studies in which experimental groups were formed only by familiar fish (e.g. Chivers et al. 1995; Bhat and Magurran 2006) living in the same aquarium for long periods such as 20 days (Seppä et al. 2001). Does this situation reflect those occurring in nature? Many fish species do not form stable shoals (e.g. Croft et al. 2004; Kelley et al. 2011) and often require several weeks to develop familiarity (Griffiths and Magurran 1997a, b; Seppä et al. 2001). Evidence in the laboratory (Lucon-Xiccato et al. 2019) and observations in nature of individuals splitting from their group in the vegetation of the canals in the Venice Lagoon suggests that the same may occur for the Mediterranean killifish. In addition, one study suggests that individuals might have difficulties at recognising social companions in large shoals because of the high cognitive effort involved (Griffiths and Magurran 1997a, b). Because of these factors, it seems reasonable that natural fish shoals are often formed by a mix of familiar and unfamiliar individuals. Therefore, at least for some species, natural shoals might be comparable to the mixed-shoal condition of our study, in which the effects of familiarity were not present or at least attenuated.
The conclusions of our study call for more research on the evolution of familiarity. If the effects of familiarity are reduced in mixed-shoals, and if this type of shoal is common in nature, one can speculate that the fitness benefits of familiarity proposed by many studies (e.g. Chivers et al. 1995; Swaney et al. 2001; Griffiths et al. 2003; Hart et al. 2014) might occur seldomly. In this light, what are the selective pressures favouring the evolution of familiarity? First, it is important to consider that we investigated only three behavioural traits. Other behaviours (e.g. Dugatkin and Alfieri 1991; Swaney et al. 2001) might be affected by familiarisation also in mixed shoals, thereby determining conditions for selection on familiarity. A more comprehensive knowledge of multiple behavioural traits in shoals formed by both familiar and unfamiliar individuals is required to fully disclose this issue.
A second aspect deserving attention is that we used relatively small shoals. Our shoal size matched those used in prior studies (Griffiths and Magurran 1999; Bhat and Magurran 2006; Davis et al. 2017; Cattelan et al. 2019) and was expected to describe at least in part the behaviour of a natural shoal (Griffiths and Magurran 1998). However, shoal size has large effects on fish behaviour (e.g. Day et al 2001; Ward and Webster 2019) and the effects reported in our study might vary according to shoal size. For instance, the disruptive effect of having unfamiliar fish in the shoal may be substantially reduced when the fraction of unfamiliar individuals is relatively small (Wilson et al. 2019). Mechanisms that increase the proportion of familiar individuals in the same shoal—even if the shoal is not completely formed by familiar individuals—might therefore provide selective pressure for familiarity. These mechanisms could be the behavioural preferences for familiar individuals (Frommen and Bakker 2004; Frommen et al. 2007; Gómez-Laplaza and Fuente 2007) and the multi-modal sensory recognition of familiar individuals, including sight, smell, and even face recognition (Griffiths and Magurran 1999; Hotta et al. 2017). An alternative mechanism to consider is that in nature, large shoals formed by both familiar and unfamiliar fish might split in two shoals with similar familiarity level. Altogether, these mechanisms might be sufficient for determining selection for familiarity.
Last, a number of co-occurring factors were not investigated in our study. For instance, we tested only females to simplify the study system. However, we know from early experiments conducted in the Mediterranean killifish that also sex ratio affects shoal behaviour and familiarity (Lucon-Xiccato and Griggio 2017; Lucon-Xiccato et al. 2017), and the same has been reported in other teleosts (Griffiths and Magurran 1998). We also known that in our study, species shoal choice based on familiarity is overridden by choice based on individual’s colouration pattern (Cattelan and Griggio 2020). Including these and other factors of variability will provide a clearer description of the behaviour of natural shoals and the potential causes for the evolution of familiarity.
In conclusion, this study suggests that the behavioural effects of familiarity may be disrupted by the presence of unfamiliar individuals in the shoal. Positive effects of familiarity (Kareem and Barnard 1982; Sackett and Fredrickson 1987; Wilkinson et al. 2010; Gutmann et al. 2015) and mechanisms that potentially introduce novel, unfamiliar individuals in the group, such as fission–fusion processes (Kerth et al. 2006; Lewis et al. 2011; Silk et al. 2014; Loretto et al. 2017), have been also observed in other vertebrate groups such as mammals and birds. Therefore, further studies should address how familiarisation affects mixed groups behaviour outside fish.
Data availability
Data were submitted as Supplementary material.
References
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. arXiv preprint:1406.5823
Bhat A, Magurran AE (2006) Benefits of familiarity persist after prolonged isolation in guppies. J Fish Biol 68:759–766
Brown C, Irving E (2014) Individual personality traits influence group exploration in a feral guppy population. Behav Ecol 25:95–101
Cattelan S, Griggio M (2020) Within-shoal phenotypic homogeneity overrides familiarity in a social fish. Behav Ecol Sociobiol 74:48
Cattelan S, Lucon-Xiccato T, Pilastro A, Griggio M (2019) Familiarity mediates equitable social associations in guppies. Behav Ecol 30:249–255
Chivers DP, Brown GE, Smith RJF (1995) Familiarity and shoal cohesion in fathead minnows (Pimephales promelas): implications for antipredator behaviour. Can J Zool 73:955–960
Cote J, Fogarty S, Brodin T, Weinersmith K, Sih A (2011) Personality-dependent dispersal in the invasive mosquitofish: group composition matters. Proc R Soc Lond B 278:1670–1678
Croft DP, Krause J, James R (2004) Social networks in the guppy (Poecilia reticulata). Proc R Soc Lond B 271:S516–S519
Davis S, Lukeman R, Schaerf TM, Ward AJ (2017) Familiarity affects collective motion in shoals of guppies (Poecilia reticulata). R Soc Open Sci 4:170312
Day RL, MacDonald T, Brown C, Laland KN, Reader SM (2001) Interactions between shoal size and conformity in guppy social foraging. Anim Behav 62:917–925
Dugatkin LA, Alfieri M (1991) Guppies and the TIT FOR TAT strategy: preference based on past interaction. Behav Ecol Sociobiol 28:243–246
Frommen JG, Bakker TCM (2004) Adult three-spined sticklebacks prefer to shoal with familiar kin. Behaviour 141:1401–1409
Frommen JG, Mehlis M, Brendler C, Bakker TCM (2007) Shoaling decisions in three-spined sticklebacks (Gasterosteus aculeatus)—familiarity, kinship and inbreeding. Behav Ecol Sociobiol 61:533–539
Galhardo L, Vitorino A, Oliveira RF (2012) Social familiarity modulates personality trait in a cichlid fish. Biol Lett 8:936–938
Gómez-Laplaza LM, Fuente A (2007) Shoaling decisions in angelfish: the roles of social status and familiarity. Ethology 113:847–855
Griffiths SW, Magurran AE (1997a) Familiarity in schooling fish: how long does it take to acquire? Anim Behav 53:945–949
Griffiths SW, Magurran AE (1997b) Schooling preferences for familiar fish vary with group size in a wild guppy population. Proc R Soc Lond B 264:547–551
Griffiths SW, Magurran AE (1998) Sex and schooling behaviour in the Trinidadian guppy. Anim Behav 56:689–693
Griffiths SW, Magurran AE (1999) Schooling decisions in guppies (Poecilia reticulata) are based on familiarity rather than kin recognition by phenotype matching. Behav Ecol Sociobiol 45:437–443
Griffiths S, Höjesjö J, Johnsson J (2003) Familiarity confers anti-predator and foraging advantages on juvenile brown trout. J Fish Biol 63:226–226
Griffiths SW, Brockmark S, Höjesjö J, Johnsson JI (2004) Coping with divided attention: the advantage of familiarity. Proc R Soc Lond B 271:695–699
Gutmann AK, Špinka M, Winckler C (2015) Long-term familiarity creates preferred social partners in dairy cows. Appl Anim Behav Sci 169:1–8
Hart PJ, Bergman E, Calles O et al (2014) Familiarity with a partner facilitates the movement of drift foraging juvenile grayling (Thymallus thymallus) into a new habitat area. Environ Biol Fish 97:515–522
Hotta T, Satoh S, Kosaka N, Kohda M (2017) Face recognition in the Tanganyikan cichlid Julidochromis transcriptus. Anim Behav 127:1–5
Jones NA, Webster MM, Salvanes AGV (2021) Physical enrichment research for captive fish: time to focus on the DETAILS. J Fish Biol 99:704–725
Kareem AM, Barnard CJ (1982) The importance of kinship and familiarity in social interactions between mice. Anim Behav 30:594–601
Kelley JL, Morrell LJ, Inskip C, Krause J, Croft DP (2011) Predation risk shapes social networks in fission-fusion populations. PLoS ONE 6:e24280
Kerth G, Ebert C, Schmidtke C (2006) Group decision making in fission–fusion societies: evidence from two-field experiments in Bechstein’s bats. Proc R Soc Lond B 273:2785–2790
Lewis JS, Wartzok D, Heithaus MR (2011) Highly dynamic fission–fusion species can exhibit leadership when traveling. Behav Ecol Sociobiol 65:1061–1069
Loretto MC, Schuster R, Itty C, Marchand P, Genero F, Bugnyar T (2017) Fission-fusion dynamics over large distances in raven non-breeders. Sci Rep 7:308
Lucon-Xiccato T, Griggio M (2017) Shoal sex composition affects exploration in the Mediterranean killifish. Ethology 123:818–824
Lucon-Xiccato T, Mazzoldi C, Griggio M (2017) Sex composition modulates the effects of familiarity in new environment. Behav Process 140:133–138
Lucon-Xiccato T, Anastasia N, Mazzoldi C, Griggio M (2019) Familiarity and sex modulate size-dependent following behaviour in the Mediterranean killifish. Sci Nat 106:31
Magnhagen C (2012) Personalities in a crowd: what shapes the behaviour of Eurasian perch and other shoaling fishes? Curr Zool 58:35–44
Maltagliati F (1998) A preliminary investigation of allozyme genetic variation and population geographical structure in Aphanius fasciatus from Italian brackish-water habitats. J Fish Biol 52:1130–1140
Maltagliati F (1999) Genetic divergence in natural populations of the Mediterranean brackish-water killifish Aphanius fasciatus. Mar Ecol Prog Ser 179:155–162
Nadler LE, McCormick MI, Johansen JL, Domenici P (2021) Social familiarity improves fast-start escape performance in schooling fish. Commun Biol 4:897
Pinheiro J, Bates D, DebRoy S, Sarkar D, Heisterkamp S, Van Willigen B, Maintainer R (2017) Package ‘nlme’. Linear and nonlinear mixed effects models, http://cran.rapporter.net/web/packages/nlme/nlme.pdf
R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
Sackett GP, Fredrickson WT (1987) Social preferences by pigtailed macaques: familiarity versus degree and type of kinship. Anim Behav 35:603–606
Seppä T, Laurila A, Peuhkuri N, Piironen J, Lower N (2001) Early familiarity has fitness consequences for Arctic char (Salvelinus alpinus) juveniles. Can J Fish Aquat Sci 58:1380–1385
Silk MJ, Croft DP, Tregenza T, Bearhop S (2014) The importance of fission–fusion social group dynamics in birds. Ibis 156:701–715
Swaney W, Kendal J, Capon H, Brown C, Laland KN (2001) Familiarity facilitates social learning of foraging behaviour in the guppy. Anim Behav 62:591–598
Utne-Palm AC, Hart PJ (2000) The effects of familiarity on competitive interactions between threespined sticklebacks. Oikos 91:225–232
Ward AJW, Hart PJB (2003) The effects of kin and familiarity on interactions between fish. Fish Fish 4:348–358
Ward AJW, Webster MM (2019) Mid-sized groups perform best in a collective decision task in sticklebacks. Biol Lett 15:20190335
Webster MM, Hart PJB (2006) Subhabitat selection by foraging threespine stickleback (Gasterosteus aculeatus): previous experience and social conformity. Behav Ecol Sociobiol 60:77–86
Webster MM, Laland KN (2012) Social information, conformity and the opportunity costs paid by foraging fish. Behav Ecol Sociobiol 66:797–809
Wechkin S (1975) Social familiarity and nip dominance in male swordtails (Xiphophorus helleri) and platys (Xiphophorus maculatus). Psychol Rep 37:435–438
Wilkinson A, Specht HL, Huber L (2010) Pigeons can discriminate group mates from strangers using the concept of familiarity. Anim Behav 80:109–115
Wilson AD, Burns AL, Crosato E, Lizier J, Prokopenko M, Schaerf TM, Ward AJW (2019) Conformity in the collective: differences in hunger affect individual and group behavior in a shoaling fish. Behav Ecol 30:968–974
Whitehead P, Bauchot JP, Hureau ML, Nielsen JC, Tortonese EJ (eds) (1986) Fishes of the North-Eastern Atlantic and the Mediterranean, vol II. Unesco, Paris
Acknowledgements
We are thankful to the reviewers for their constructive comments and to the students of the “Biodiversity and Behaviour” class at the Marine Biology Course, University of Padova, for helping during the experiments. This article is dedicated to the memory of MG, who passed away few days after completing the manuscript.
Funding
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement. Funding was provided by PRAT grant (n. PRAT-2015–297-CPDA153859) from Università di Padova to MG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Experiments were conducted in accordance with the law of the country in which they were performed (Italy, D.L. 4 Marzo 2014, n. 26). The Ethical Committee of Università di Padova reviewed and approved all the experimental procedures (protocol n. 1098). No physical invasive manipulations were performed on the fish during the experiments and no fish showed sign of distress. At the end of the experiments, all subjects were released at the sampling place.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by J. G. Frommen.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic Supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lucon-Xiccato, T., Cattelan, S. & Griggio, M. Familiarity effects on fish behaviour are disrupted in shoals that contain also unfamiliar individuals. Behav Ecol Sociobiol 76, 100 (2022). https://doi.org/10.1007/s00265-022-03210-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03210-9