Abstract
The evolution of obligate kleptoparasitism, the theft of food, has led to remarkable innovations, including physical weapons and chemical signals that can evolve into chemical weapons. Stingless bees in the genus Lestrimelitta are excellent examples of this phenomenon because they are obligate kleptoparasites that no longer collect floral resources and instead steal brood resources from other bees. Their ability to raid successfully is thus essential to their fitness even when they fight species that are physically bigger, have larger defense forces, or both. We conducted morphometric analyses, quantified Lestrimelitta niitkib mandibular gland pheromone (MGP) components, and carried out individual fighting trials between L. niitkib and the stingless bee Scaptotrigona mexicana, a common victim species, to shed light on the detailed reasons for their success at robbing. Measurements showed that L. niitkib mandibles have thicker exoskeleton cuticles and overall greater width, particularly in the medial and proximal sections, than S. mexicana, which is quite similar in body size. In all fights, L. niitkib bit victims and released MGP, as it does during raids. Scaptotrigona mexicana victims exhibited significantly increased uncoordinated behaviors and showed partial or complete paralysis. We analyzed and quantified the major components of MGP, which consisted of large quantities of geranial (mean of 253 μg) and neral (48 μg) per bee. Microinjections of 1 bee equivalent (BE) of natural or synthetic MGP and ≥ 0.1 BE of geranial significantly increased deleterious behaviors and paralysis as compared to control injections. We suggest that the large quantities of MGP used during raiding have led to an unexpected outcome, a semiochemical evolving the additional function of a toxin, and contribute to the ability of Lestrimelitta to rob its victims.
Significance statement
Kleptoparasites, organisms that steal food resources, employ multiple physical and chemical tools to survive. The success of kleptoparasitism requires a balance between honesty and coercion in interspecific communication. The genus Lestrimellita consists of a group of kleptoparasitic stingless bee species that raid other bee colonies for food and therefore depend upon winning these raids. However, why they succeed remains not fully understood. We studied differences in morphology between L. niitkib and its victims, the pheromones they release during raids, and ran individual fight trials between L. niitkib and a common victim to identify why they are successful. We suggest that the release of pheromones at the beginning of raids, in concert with the pheromone’s toxicity, has been combined to improve L. niitkib’s ability to successfully rob.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The roles of honesty and coercion in the evolution of chemical signals have been extensively debated (Villalta et al. 2018; Orlova and Amsalem 2019) and extended to communication between different species (Brown et al. 1970; Ruther et al. 2002; Garvey et al. 2017). The effects of such chemical signals vary with the advantages accruing to senders and receivers, and common interactions over evolutionary time should favor interspecific chemical communication, particularly if the signal is a reliable and honest indicator of predator or aggressor quality (Bradbury and Vehrencamp 1998). If the chemical signal is also a toxin (i.e., an allomone, Brown et al. 1970) such as formic acid in ant venom glands (Hölldobler et al. 1990) or in the mandibular gland secretions of Oxytrigona stingless bees (Roubik et al. 1987), signal honesty should theoretically be reinforced. This role of allomones serving as signaling compounds and toxins in animal warfare is fascinating and has usually been explored from the perspective of a toxin evolving into a signal (Rinderer et al. 1988; Hölldobler et al. 1990; Hölldobler et al. 2009). However, the toxicity of a compound also relates to the dose received (Ottoboni 1984), and thus a non-toxic signal could theoretically evolve to become toxic if produced in large quantities (Hölldobler and Engel 1978; Hölldobler et al. 2013). This potential for a signal to become venom-like is poorly understood.
For decades, researchers have been intrigued by the chemical signals and fighting abilities of Lestrimelitta (Nogueira-Neto 1970; Wittmann 1985; Johnson 1987; Sakagami et al. 1993), a genus of neotropical eusocial bees that are obligate kleptoparasites (robbers) (Müller 1874; Schwarz 1948; Michener 2000; Breed et al. 2012; Mascena et al. 2017; Guevara et al. 2020). Lestrimelitta species do not forage on flowers for nectar or pollen (Michener 2000). Instead, they steal brood resources and pollen from other bee colonies (Sakagami and Laroca 1963; von Zuben and Nunes 2014; Grüter et al. 2016) and are such formidable robbers that they have likely contributed to the soldier caste of Tetragonisca angustula and other sympatric stingless bee species (Grüter et al. 2012; Grüter et al. 2017).
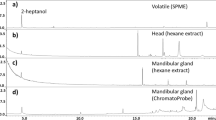
The odors produced by Lestrimelitta are of particular interest: the labial gland extracts of Lestrimelitta limao cause Frieseomelitta varia workers to retreat inside the nest (von Zuben et al. 2016), and L. niitkib scouts may partially evade detection by sharing similar cuticular hydrocarbons with a preferred host species, Nannotrigona perilampoides (Quezada-Euán et al. 2013). The behavior of F. varia workers suggests that they may be exposed to L. limao labial gland compounds during raids and thus that labial gland secretions may be used as a recruitment or raiding pheromone. Additional studies on this question should be conducted given that the labial glands, in multiple meliponine species, are used to create odor trails that help guide nestmates in rewarding food sources (Jarau and Hrncir 2009). However, attention has focused on Lestrimelitta mandibular gland pheromone (MGP) because it is produced in such copious amounts, particularly at the beginning of a raid, and elicits strong receiver responses (Sakagami et al. 1993). MGP primarily consists of two citral isomers, geranial and neral (Blum et al. 1970; von Zuben et al. 2016), and is a raiding pheromone whose primary function is evidently to recruit Lestrimelitta swarms to victim colonies (Blum 1966; Sakagami et al. 1993). MGP has also been called an alarm pheromone, but its function at Lestrimelitta nests is largely unknown (Grüter 2020). Sakagami and Laroca (1963) note that L. limao nests are normally constantly saturated with the odor of MGP, which does not support the alarm pheromone hypothesis unless colonies are in a perpetual state of alarm. We therefore refer to L. niitkib mandibular gland pheromone simply as MGP, not as an alarm pheromone, and recognize that it is a raiding pheromone because of its close association with raids.
Almost all studies have focused on the effects of MGP at victim colonies, where the responses of victims may be adaptive, a result of manipulation by the robbers, or both (Grüter 2020). MGP has been hypothesized to disorient victims by overloading their olfaction (Blum et al. 1970; Sakagami et al. 1993), to cause victims to flee (Pompeu and Silveira 2005), to mask the ability of victims to perceive their own alarm pheromones (supersedure hypothesis) and coordinate defense (Johnson 1987; Kerr 1951; Moure et al. 1958), to disrupt victim social behaviors (Blum et al. 1970), to tranquilize and induce victim submission (Sakagami et al. 1993), or to induce victim torpor (toxin effect) (Sakagami et al. 1993). The toxin hypothesis is also proposed by Nogueira-Neto (1970), who observed L. limao licking dead or dying Plebeia droryana victims and found that bees remained momentarily motionless after a L. limao bite. MGP may have all these functions, but Lestrimelitta’s raiding success is most likely related to their prowess as robbers. Lestrimelitta can repeatedly attack the same victim colonies (Sakagami et al. 1993), and victims should therefore be able to associate MGP with imminent raiding (Campollo-Ovalle and Sánchez 2018) and chose the best response: fleeing (Pompeu and Silveira 2005), aggressively counter-attacking (Nunes et al. 2014; von Zuben and Nunes 2014), or using other methods to resist (Sakagami et al. 1993).
Lestrimelitta must successfully raid to survive and therefore usually wins, even when victim species are physically larger bees, have colonies with a defense force that outnumbers Lestrimelitta raiding parties, or both (Sakagami et al. 1993). The ability of L. limao foragers to dominate may arise from multiple traits such strength, endurance, or increased robustness of their mandibles, the main physical weapon of stingless bees (Roubik 1992). In ants, changes in jaw morphology and robustness, as dramatically illustrated by soldier castes, facilitate the cutting or crushing of enemies (Helanterä and Ratnieks 2008; Hölldobler et al. 1990). Biting plays a key role in L. limao raids (Nogueira-Neto 1970; Grüter et al. 2012), particularly when they attack Scaptotrigona postica (Sakagami et al. 1993). Biting is the main offensive and defensive strategy of stingless bees (Roubik 1992) and allows Oxytrigona species to spread their toxic mandibular gland pheromone (MGP) onto victim bodies and into the bite wound (Roubik et al. 1987; Rinderer et al. 1988). In some meliponine species, defenders bite and do not disengage, even at the cost of suffering fatal damage (Shackleton 2015).
Lestrimelitta niitkib shares the raiding behavior of other Lestrimelitta and is found throughout the Yucatan Peninsula (Quezada-Euán and González-Acereto 2002) where it is sympatric with and commonly attacks Scaptotrigona mexicana (Quezada-Euán and González-Acereto 2002). In preliminary fight trials, we noticed that S. mexicana victims would die despite no loss of limbs and relatively inconspicuous puncture wounds. Thus, we tested the fighting ability of L. niitkib in two ways: honest fighting ability and the toxicity of its MGP to victims. We measured and compared the mandibles of L. niitkib and two of its common victims, S. mexicana and T. angustula. We then conducted paired fighting trials between individual L. niitkib raiders and S. mexicana guards and recorded behaviors such as the number of falls, the number of paralyzed limbs, and the number of attempts to fly. Using gas chromatography-mass spectrometry (GC-MS), we chemically analyzed and quantified the major components of L. niitkib’s MGP and then measured the effects of natural pheromone, synthetic pheromone, and individual synthetic pheromone components at one bee equivalent or less applied via injection to S. mexicana guards.
Methods
Colonies and study site
We conducted our study on the campus of El Colegio de la Frontera Sur (ECOSUR) in Tapachula, Chiapas, Mexico. Although L. niitkib is widespread, it is relatively rare (Roubik 1992). We obtained two natural L. niitkib colonies in trees from the surrounding area. For each colony, we cut down the tree to obtain a trunk section that housed the colony. Colonies were placed on opposite sides of the ECOSUR campus and used for our studies after they had acclimated to their new location for over 1 year. We never observed the L. niitkib colonies raiding each other. Victims consisted of six S. mexicana colonies originally found nesting in trees around Tapachula and transferred to wood hives on the ECOSUR campus where they lived for over 1 year before we conducted our experiments. Sample sizes are given in the figure legends.
Mandible comparisons
To compare mandibular morphology, we randomly selected the right or left mandible of freeze-killed L. niitkib, S. postica, and T. angustula guard bees (using only one mandible per bee) and used fine dissecting forceps to detach it with from the head capsule at its joint. Guards were identified as bees standing at the nest entrance and gaping their mandibles in response to an approaching human. We measured mandible exoskeleton (cuticle) thickness with scanning electron microscopy (Topcon SM-510 scanning electron microscope, Singapore, Singapore). For mandible cuticle thickness measurements, one mandible per bee (left or right, randomly selected) was cut open with a razor at the distal point near the biting edge, imaged with scanning electron microscopy, and then measured (Fig. 1a, b). Using a separate set of bees, we measured mandibular widths with a Zeiss Axio Imager research microscope (Jena, Germany). We used one mandible per bee (left or right, randomly selected) and took calibrated photos to measure the proximal (near head), medial, and distal (near the biting edge) widths. We also measured the intertegular distance (the distance between the attachment points of the wings on the thorax), a standard allometric measurement for comparing bee sizes (Kendall et al. 2019) (Fig. 1).
Morphology of stingless bee mandibles. a Violin plots comparing mandible cuticle thicknesses of L. niitkib and two victim species (S. mexicana and T. angustula). Thickness was measured at distal width point because this is close to the biting edge. Different letters indicate significant differences (Tukey’s HSD test, P < 0.05). We used one mandible per bee from 7 L. niitkib (from 2 colonies), 12 S. mexicana (6 colonies), and 11 T. angustula guards (6 colonies). b Plot of mean mandible cuticle thickness vs. a standard allometric measure of bee size (intertegular distance). Error bars show ± 1 standard deviation in both mean mandible thickness and intertegular distance per species c scanning electron micrographs of mandibles of the three species (the distal “cutting” edge faces the right). d Principal component analysis of the three mandible width measurements. Inset T. angustula mandible shown with distal (D), medial (M), and proximal (P) widths indicated. We used one mandible per bee from 28 L. niitkib (2 colonies), 35 S. mexicana (6 colonies), and 20 T. angustula (6 colonies) guards. Colors represent species and ellipses that show a 95% standard error around each species’ distribution. Percentages on the axes indicate the proportion of variation that the axis represents. Vectors (arrows) indicate the degree to which each measurement variable (medial, proximal, and distal width) drives variability along a given principal coordinate. e Plot of mean mandible width (an average of medial, proximal, and distal widths per individual per species) vs. intertegular distance. Error bars show ± 1 standard deviation in both mean mandible width and intertegular distance per species
Fight trials
Fights were conducted between L. niitkib and S. mexicana because they are a similar size to one another (Fig. 1, intertegular distance), and we wanted to compare the relative, one-on-one fighting capabilities of L. niitkib with victim species. To determine fight outcomes, we paired one L. niitkib forager with one S. mexicana guard. We obtained these bees by approaching their respective nest entrances with a clean glass vial and capturing one L. nittkib worker as it departed to forage and, in a separate vial, one S. mexicana guard that defensively gaped its mandibles and flew at the vial. The vials were then capped with clean cotton and immediately brought back into the lab for testing. In the lab (about 1 min after capture), we removed the cotton and checked for the odors of L. niitkib MGP and S. mexicana alarm pheromone. The odor of S. mexicana alarm pheromone is quite easily detected and learned by observers when guards from two different S. mexicana colonies are brought together and fight. Lestrimelitta that released MGP or S. mexicana that discharged alarm pheromone were not used because we wished to use only bees that were “fit-to-fight” and that were not damaged, weakened, or alarmed during the capture process. In general, < 1% of bees captured were not used because they had discharged MGP or alarm pheromone. In addition, we observed that L. niitkib guards seldom elicit defensive behavior or produce MGP even when attacked at their nest entrance (matching what Sakagami et al. 1993 reported for L. limao).
We then brought both open vial ends together and briefly and lightly agitated the vials to bring the bees together. If no fighting occurred after 3 min, the trial ended, bees were chilled to reduce their motion, painted with permanent acrylic paint on their thoraces to ensure that they would not be reused, and then released. We also painted and then released bees that were captured and not used in a fight trial. Once an attack occurred, defined as the bees grappling or biting each other, we allowed the attack to continue for 5 s before gently and carefully separating the bees with entomological forceps and placing them in small petri dishes (35-mm diameter × 10-mm high). The bees were then observed for 20 min. We recorded the following: presence or absence of L. niitkib MGP release (easily detected because of its strong, characteristic odor), if a bee was bitten (we examined bees with a Zeiss Stemi 518 stereo microscope, Germany), if a leg or wing was cut off, the number of limbs or wings paralyzed (defined as the appendage remaining motionless for the remainder of the trial), the number of falls, and the total time spent motionless. Based upon preliminary trials, we defined a fall as a bee flipping over and remaining on its thorax for ≥ 3 s. In control trials, individuals were identically captured and separately agitated, as in the fight trials, but they never came into contact and were then placed in identical separate petri dishes (as above) and observed for 20 min. No bees died in any of these control trials.
Chemical analysis of MGP
Neral and geranial were synthetized in good yields (95% and 94%, respectively) from their respective alcohols (nerol and geraniol) by Corey’s oxidation with SiO2 (Fernandes and Kumar 2003; Luzzio et al. 1999) to obtain standards for Gas Chromatography-Mass Spectrometry (GC-MS) analyses. For this procedure, pyridinium chlorochromate was freshly prepared. Pure aldehydes were obtained after purification of the crude extracts with flash column chromatography using hexane-acetone (95:5) as the eluent. All chemicals were purchased from Sigma-Aldrich.
To obtain MGP, L. niitkib foragers were collected from nest entrances as described above. We only used bees that did not release MGP during capture. The mandibular glands (two glands per bee) of six foragers from colony one and four foragers from colony two were carefully dissected out under a stereoscopic microscope and then macerated with 1 mL of hexane for 5 min. We used only glands that were not punctured during dissection. Each sample consisted of both glands from one bee. The extracts were concentrated using a gentle stream of dry N2 to a volume of 400 μl per sample and were stored in a −20 °C freezer until analysis.
Extracts were analyzed on a GC-MS Varian Star model 3400 CX GC (Palo Alto, CA, USA). A DB-5 column (30 m × 0.25 mm ID) was temperature-programmed from 50 °C (held for 2 min) to 280 °C at 15 °C min−1 and held at 280 °C for 10 min. The temperature of the injector was 250 °C. This GC was coupled to a Varian Saturn 4D mass spectrometer and integrated data system. Ionization was carried out by electron impact at 70 eV and 250 °C. Compounds were verified and quantified with the pure, previously synthesized neral and geranial standards (see above). Pheromone components were quantified by measuring the area under each peak in comparison with external standard curves. To prepare the calibration curves, neral was diluted to 3 ng/μL, 9 ng/μl, 14 ng/μl, 29 ng/μl, 57 ng/μl, 287 ng/μl, and 574 ng/μl. Geranial was diluted to 6 ng/μl, 17 ng/μl, 28 ng/μl, 56 ng/μl, 112 ng/μl, 557 ng/μl, and 1114 ng/μl.
Testing the toxicity of MGP
During fights, we often observed L. niitkib using its mandibles to cut and puncture S. mexicana, as described by Sakagami et al. (1993) for L. limao attacking S. postica. Following fights, victims smelled strongly of L. niitkib MGP. In preliminary trials, we attempted to measure the amount of MGP that L. niitkib could inject or apply to its victims via cuts after the 3 min fight trial but were unfortunately unable to quantify this with our GC-MS apparatus. We therefore conducted injection trials to explore the toxicity of L. niitkib MGP on S. mexicana and to test the hypothesis that MGP can be toxic (see discussion on citral in Sakagami et al. 1993). We injected each S. mexicana guard with 1 μl of insect Ringer’s solution (Yamasaki and Narahashi 1959) containing the treatment. We used the following treatments: a control (Ringer’s solution only), 1 bee-equivalent (BE) of natural MGP extract, 1 BE of synthetic MGP (geranial and neral in a 5.25:1 natural ratio), 1 BE of pure synthetic neral, and 1, 0.5, or 0.1 BE of geranial. Natural MGP extracts were collected from dissected mandibular glands as described for MGP chemical analysis (see above). We did not test lower levels of pure neral because 1 BE of neral had no effect (see “Results”). However, we tested the effects of lower geranial levels because 1 BE of geranial significantly impaired S. mexicana guards.
For these trials, guard bees were captured in glass vials as previously described. For the injections, they were transferred into a queen marking cage (cylinder 30-mm diameter × 80-mm long) with a soft foam-covered plunger (Dadant and Sons, Fresno, CA, USA) and modified by using a mesh cover on one side. Bees were placed into the opening and the plunger was inserted into the tube until the bee’s dorsal side gently rested against the mesh. This allowed bees to be injected with solutions without being chilled, because chilling increased mortality in preliminary trials. Bees were injected in the abdomen with a fine Hamilton syringe (#701, injection depth of approximately 0.1 mm) and then placed in a small plastic petri dish (35-mm diameter × 10-mm high) and observed for 20 min, a duration chosen, because we observed few behavioral changes after 20 min in preliminary trials. As in the fight trials, we recorded the number of falls, the time spent moving, the rate of falls per time spent moving, and the total time spent motionless. Because these compounds (particularly at 1 BE) have a strong odor that humans can detect following injection, these injections were not performed blind. We therefore used specific, clear behavioral measures designed to minimize potential observer bias.
Statistical analyses
To test for differences in mandible thickness, we used a mixed model (REML algorithm) with species as a fixed effect and colony as a random effect. To test for differences between mandible widths, we used a Repeated Measures mixed model (REML algorithm) with Bee ID nested within species, species and measurement type as fixed effects, and the interaction species x measurement. Colony was a random effect. We used Tukey’s honestly significant difference (HSD) tests to make multiple pairwise comparisons.
For the fight and injection data, we used two different models. For nominal variables (bitten during a trial, at least one limb cutoff during a trial, or tries to fly during a trial), we used nominal logistic regression, reporting our results as likelihood ratio (LR) chi-square tests. For the fight data, we separately analyzed data for each trial type (fight or no-fight). For fight and injection trials, we used REML algorithm to analyze continuous variables with treatment as a fixed effect and S. mexicana colony as a random effect. To analyze the injection data, we log-transformed the number of falls, number of falls per time in motion, and time spent motionless. We used Tukey’s HSD tests to make multiple pairwise comparisons. We applied the Dunn-Sidak correction to our analyses of the number of falls and the rate of falls (k = 2) because the falling rate was calculated from the data on falls, and we denote significant P-values as DS. Analyses were run with JMP Pro v11 software. To compare deaths between the two species during fight trials, we used a 2 × 2 two-tailed Fisher’s exact test (https://www.graphpad.com/quickcalcs/contingency2/).
Violin plots were used to show the distributions of our mandible morphology results (Fig. 1) and injection trials (Fig. 4) as we felt these better captured the distributions and variability in the data. Violin plots differ from boxplots in that they show the density of data across the variable axis through the width of the “violins.”
Data availability statement
All data is available on Zenodo.org, DOI: 10.5281/zenodo.5646494.
Results
Mandible comparisons
Lestrimelitta niitkib (26.5-μm mean mandibular exoskeleton thickness) have mandibles with nearly twice the exoskeleton cuticle thickness of T. angustula (14.5 μm) or S. mexicana (14.6 μm) mandibles (F2,51 = 73.456, P < 0.0001, Tukey’s HSD test, P < 0.05, Fig. 1a). In terms of mandibular widths (Fig. 1c), the species were significantly different (F2,79 = 1446.8, P < 0.0001); there was a significant effect of measurement location (F2,160 = 1606.5, P < 0.0001) and a significant interaction of species x measurement location (F4,160 = 306.7, P < 0.0001). In detail, L. niitkib mandibles have significantly greater proximal and medial widths than the other two species (Tukey’s HSD test, P < 0.05). However, for distal widths, S. mexicana > L. nittkib > T. angustula (Tukey’s HSD test, P < 0.05). Principal component analysis reveals a clear separation of mandibular width measurements from the three species (Fig. 1d). In addition, L. nittkib had thicker mandibular cuticles (Fig. 1b) and wider average mandible widths (distal, medial, and proximal widths averaged per individual and then per species, Fig. 1e) than S. mexicana, which is very similar in body size based upon a common allometric measure of bee body size, the intertegular distance (L. nittkib 1.51 ± 0.02 mm, S. mexicana 1.48 ± 0.03 mm).
Fight trials
We analyzed 21 no-fight trials and 32 fight trials with S. mexicana from four colonies and L. niitkib from two colonies. We detected the characteristic odor of L. niitkib MGP on the bodies of S. mexicana victims in all experimental trials but not in any of the control trials. In our 53 fight trials, 13 S. mexicana and 2 L. niitkib died during the 20-min trial (Fisher’s exact test, P = 0.004).
After fight trials, L niitkib guards were more active and tried to fly significantly more often than S. mexicana (4.5-fold more, χ21 = 30.0, P < 0.0001, Fig. 2a). For no-fight trials, there was no significant difference between L. niitkib and S. mexicana attempts to fly (χ21 = 0, P = 1.0, Fig. 2a). In all fight trials (100%), S. mexicana was bitten by L. niitkib. However, L. niitkib was bitten by S. mexicana in 87.5% of trials (significantly less, χ21 = 5.8, P = 0.016). No bees were bitten in no-fight trials. In fight trials, S. mexicana was nearly 12-fold more likely to have a leg or wing cutoff than L. niitkib (χ21 = 13.3, P = 0.0003, Fig. 2a).
Results from fight (experimental, n = 64) and no-fight (control, n = 42) trials. a Behavioral responses and outcomes scored per trial (yes/no data) for L. niitkib (2 colonies) and S. mexicana (4 colonies). No bees were bitten or had limbs removed in the control trials. Asterisks indicate significant differences (P ≤ 0.016) between species per trial type (“ns” indicates no significant difference). In the no-fight trials, no statistical tests were conducted for being bitten, or having a leg or wing cutoff because none of these behaviors or outcomes occurred. Boxplots show b time spent motionless (s), c the number of falls/time spent in motion, and d the number of paralyzed legs or wings per trial (different letters indicate significant differences, Tukey’s HSD tests, P < 0.05)
For time spent motionless, there were significant effects of trial type (F1,100 = 74.2, P < 0.0001), species (F1,99 = 65.2, P < 0.0001), and the interaction trial type x species (F1,99 = 65.2, P < 0.0001) because S. mexicana spent 24.2-fold more time motionless than L. niitkib in fight trials (Fig 2b). In non-fight trials individuals of both species did not spend any time spent motionless. Scaptotrigona mexicana colony identity accounted for < 1% of model variance.
In fight trials, S. mexicana had 4.7-fold higher falling rate (falls per time spent in motion) than L. niitkib (Fig. 2c, Tukey’s HSD test, P < 0.05). There were significant effects of species (F1,99 = 11.5, P = 0.001), trial type (F1,101 = 10.8, P = 0.001), and the interaction species x trial type (F1,99 = 35.5, P < 0.0001). Scaptotrigona mexicana colony identity accounted for < 1% of model variance. Finally, in fight trials, S. mexicana guards had 16-fold more paralyzed legs and wings than L. niitkib (a significant difference, Tukey’s HSD test, P < 0.05): significant effects of species (F1,97 = 7.4, P = 0.008), trial type (F1,100 = 9.5, P = 0.003), and the interaction species x trial type (F1,97 = 7.43, P = 0.008, Fig. 2d). Scaptotrigona mexicana colony identity accounted for 11% of model variance.
L. niitkib MGP analysis
We used GC-MS, to determine the chemical composition of L. niitkib MGP (Fig. 3). Guards from both colonies had similar amounts and ratios of geranial and neral per bee (colony one 0.282 ± 0.054 μl geranial, 0.056 ± 0.003 μl neral, geranial/neral ratio = 5.06; colony two 0.282 ± 0.032 μl geranial, 0.055 ± 0.003 μl neral, genial/neral ratio = 5.18). Overall, each bee had an average of 0.283 μl (253 μg) of geranial and 0.054 μl (48 μg) of neral (g/n ratio of 5.25 ± 0.56:1). We therefore used these volumes as our 1 bee equivalent (BE) standards in the injection trials.
Injection trials
In total, we injected 159 bees from six S. mexicana colonies. For each injection type, we used 20 bees (except for natural MGP extracts from colony two, which used 19 bees). The injection of natural MGP treatment significantly increased adverse outcomes as compared to the control injection of Ringer’s solution only. Each of the following increased with increasing MGP doses: number of falls (F6,148 = 35.2, P < 0.0001 DS, 2% colony effect, Fig 4a), time spent motionless (F6,148 = 3.6, P = 0.0021, 4% colony effect, Fig. 4b), and the rate of falls per time spent moving (fall/min, F6,149 = 19.8, P < 0.0001 DS, <1% colony effect, Fig 4c). All treatments, except 1 BE of neral, significantly increased the number of falls as compared to the control (Tukey’s HSD test, P < 0.05). Likewise, 1 BE of natural or synthetic MGP significantly increased the time spent motionless in comparison to the control (Tukey’s HSD test, P < 0.05). Finally, the rate of falls per time spent in motion was significantly higher for 1 BE of natural and synthetic MGP and 1 BE or 0.5 BE of geranial as compared to the control (Tukey’s HSD test, P < 0.05). In all measurements, neral did not significantly alter behavior as compared to the control treatment (Tukey’s HSD test, P < 0.05). Natural and synthetic MGP had the same effects, and geranial was the only MGP component that significantly increased falls and the rate of falls, even at 0.5 BE (Tukey’s HSD tests, P < 0.05, Fig. 4).
Violin plots showing results of the injection trials with workers from 2 L. niitkib colonies (Natural MGP) and 6 S. mexicana colonies (injected bees). Sample sizes for each trial are as follows: 1 BE Natural MGP (n = 39), 1 BE Synthetic MGP (n = 20), 1 BE Geranial (n = 20), 0.5 BE Geranial (n = 20), 0.1 BE Geranial (n = 20), 1 BE Neral (n = 20), 1 μl Ringer’s control (n = 20). We plot a the number of falls, b the amount of time spent motionless (s), and c the number of falls per minute spent in motion (falls/min). Different letters indicate significant differences (Tukey’s HSD test, P < 0.05)
Discussion
Lestrimelitta niitkib is an obligate kleptoparasite that relies upon its fighting abilities, including the ability to recruit many raiders with its mandibular gland pheromone (MGP). Our experiments show that, even in one-on-one fights, L. niitkib are excellent fighters and that the large quantities of MGP they each produce have a previously unappreciated effect. Lestrimelitta niitkib’s biting attacks may allow the pheromone to enter through a victim’s exoskeleton and act like a toxin. Moreover, L. niitkib has formidable mandibles that have significantly thicker exoskeletons at their cutting edges and, in terms of overall width, are significantly wider proximally and medially than the mandibles of S. mexicana and T. angustula, two species that it commonly raids. A comparison with S. mexicana is revealing because L. nittkib had 1.8-fold thicker and 1.1-fold greater average mandible widths than S. mexicana, despite being quite similar in overall size. A detailed biomechanical strength analysis, particularly measuring bite force, would be useful for future studies. Unlike T. hyalinata, T. fuscipennis, or T. spinipes, we did not observe the presence of sharp teeth-like structures (Shackleton 2015) in L. niitkib mandibles. However, a straight, blade-like edge such as exhibited by L. niitkib could also exert considerable damage (Fig. 1C), as illustrated by the number of victims’ wing and leg cut off in fight trials (Fig. 2A).
In aggression trials, all S. mexicana were bitten by L. niitkib, yet significantly fewer L. niitkib (87.5%) were bitten by S. mexicana. After individual fights, S. mexicana sustained more injuries than L. niitkib: 39% of all S. mexicana lost limbs and approximately 32% had paralyzed body parts. Scaptotrigona mexicana also had 4.7-fold more falls per time spent moving than L. niitkib after fights. These injuries may have reduced flight abilities since S. mexicana tried to fly 5-fold less than L. niitkib following fights. Similarly, Grüter et al. (2012) observed that L. limao have strong mandibles and eventually won all fights against the substantially smaller T. angustula guards, often by decapitating them, although larger guards were able to fight longer.
In our fight trials, all S. mexicana guards were bitten, and all bore the strong, characteristic odor of L. niitkib MGP on their bodies. Scaptotrigona mexicana injected with one bee equivalent (1 BE) of natural or synthetic MGP fell more often, had a higher falling rate per time spent in motion, and spent more time motionless. Although 1 BE of MGP is a relatively large amount, injection with 0.1 BE of geranial also significantly increased falling (Fig. 4). However, the results of this experiment should be interpreted with caution given that even 0.1 BE of geranial could be larger than the amount typically injected into a victim. In raids upon nests, we observed that S. mexicana workers could also be attacked and bitten by multiple L. niitkib attackers. In these cases, the amount of MGP naturally injected could be higher. Quantifying the amount of MGP that typically enters a victim’s body is therefore important, although we were unable to do so with our available GC-MS apparatus. MGP is quite volatile and the amount in victim bodies could not be preserved for analysis with a more sensitive GC-MS at a different location. However, such analyses should be possible for future studies using different apparatus. Papachristoforou et al. (2012) showed that 2-heptanone in honey bee mandibular gland secretions can enter and paralyze bitten victims (wax moth larvae and Varroa mites) at measured levels of 0.65 nL per wax moth larvae (GC-MS analysis).
Kleptoparasitism is a derived trait in stingless bees (Michener 2000), and thus the ancestor to Lestrimelitta was a floral foraging social species that probably used, like other stingless bees, its mandibular gland secretions as an alarm pheromone (Schorkopf et al. 2009), not as a venom. In fact, geranial and neral are widespread in the mandibular gland pheromones of multiple stingless bee species, where they are used in alarm communication (Blum et al. 1970). These citral isomers are also simple compounds that are quite unlike most venom compounds (Casewell et al. 2013). Thus, Lestrimelitta’s dependence on recruitment raids may have favored an increase in raiding pheromone per bee that, in conjunction with its strong, piercing mandibles, may have led to dosage-based toxicity. In general, large increases in the volume of a chemical signal may contribute to toxic or defensive properties. The pygidial glands of some ants produce an alarm pheromone containing benzaldehyde, but, in some ant species, these glands can reach a fairly large size and produce quantities of benzaldehyde that act as a chemical defense (Hölldobler and Engel 1978; Hölldobler et al. 2013). The toxic effects of dose may account for why 1 BE of geranial, but not neral, harmed S. mexicana because each Lestrimelitta raider had 5-fold more geranial than neral. However, 0.1 BE geranial did significantly increase the number of falls, suggesting that geranial is more harmful than neral. Testing these different compounds in multiple other victim species would be useful for future studies. Furthermore, other chemicals such as Lestrimellita labial gland compounds should be tested: L. limao labial gland compounds (hexadecyl acetate and 9-hexadecenyl acetate) repelled Frieseomelitta varia foragers and guards (von Zuben et al. 2016) and may serve other functions.
Other stingless bee species may also have evolved to use defensive mandibular gland compounds for kleptoparasitism. The meliponine genus Oxytrigona (fire bees) secrete formic acid in their mandibular glands (Roubik et al. 1987). These secretions are typically viewed as defensive because the Oxytrigona usually forage for floral nectar and pollen (Schwarz 1948), but they are facultative kleptoparasites and can steal honey from honey bee (Apis mellifera) nests. Rinderer et al. (p496 1988) wrote, “During nest plundering, the fire bee produces a cephalic secretion which has a strong but, to humans, pleasant floral odor…honeybees do not defend their nest but remain motionless on the comb, hang in a cluster of bees outside the entrance of the colony, or appear to “wander” in a seemingly disoriented manner over the surface of the comb.” This behavior is strikingly like the actions of Lestrimelitta victims described by Sakagami et al. (1993).
Lestrimelitta raiding is therefore an excellent model for understanding a classic evolutionary problem, the role of honesty and coercion in signal evolution between an exploiter and the exploited. We propose that honesty is a more productive framework to view the functions of Lestrimelitta MGP, which is a raiding pheromone tied to Lestrimelitta’s usually superior fighting ability. As a result, MGP elicits a spectrum of responses in victim species that likely reflect a victim colony’s ability to defend itself and the learned experiences of victims with prior Lestrimelitta raids. For example, T. angustula victim colonies can adaptively increase the number of soldiers and regulate soldier body size when defending the colony from elevated levels of L. limao attacks (Segers et al. 2016). Such colony-level response plasticity should be considered in long-term interactions between Lestrimelitta colonies and those that it exploits. Similarly, the potential of MGP to server as an honest signal of Lestrimelitta’s raiding abilities could depend upon the prior experiences of victims and if they resisted or succumbed to Lestrimelitta raids. This question should be explored in future studies. However, we suggest that the large quantities of MGP produced during Lestrimelitta raids have led to an unexpected outcome: a semiochemical evolving the additional function of a toxin. If so, like formic acid or venom-associated compounds, MGP is an example of honest communication in which signal is also a toxin. This interplay of benefits and costs among senders and receivers, even across different species, is crucial for signal evolution, and deserves greater study, particularly in the context of animal warfare (McGregor 2005).
References
Blum MS (1966) Chemical releasers of social behavior. VIII. Citral in the mandibular gland secretion of Lestrimelitta limao (Hymenoptera: Apoidea: Melittidae). Ann Entomol Soc Am 59:962–964
Blum MS, Crewe RM, Kerr WE, Keith LH, Garrison AW, Walker MM (1970) Citral in stingless bees: isolation and functions in trail-laying and robbing. J. Insect Physiol 16:1637–1648
Bradbury J, Vehrencamp S (1998) Principles of animal communication. Nature 391:31–32
Breed MD, Cook C, Krasnec MO (2012) Cleptobiosis in social insects. Psyche
Brown WL, Eisner T, Whittaker RH (1970) Allomones and kairomones: transspecific chemical messengers. BioScience 20:21–21
Campollo-Ovalle A, Sánchez D (2018) Temporal response of foragers and guards of two stingless bee species to cephalic compounds of the robber bee Lestrimelitta niitkib (Ayala) (Hymenoptera, Apidae). Neotrop Entomol 47:791–797
Casewell NR, Wüster W, Vonk FJ, Harrison RA, Fry BG (2013) Complex cocktails: the evolutionary novelty of venoms. Trends Ecol Evol 28:219–229
Fernandes RA, Kumar P (2003) PCC-mediated novel oxidation reactions of homobenzylic and homoallylic alcohols. Tetrahedron Lett 44:1275–1278
Garvey PM, Glen AS, Clout MN, Wyse SV, Nichols M, Pech RP (2017) Exploiting interspecific olfactory communication to monitor predators. Ecol Appl 27:389–402
Grüter C, Menezes C, Imperatriz-Fonseca VL, Ratnieks FLW (2012) A morphologically specialized soldier caste improves colony defense in a neotropical eusocial bee. Proc Natl Acad Sci 109:1182–1186
Grüter C, von Zuben LG, Segers FHID, Cunningham JP (2016) Warfare in stingless bees. Insect Soc 63:223–236
Grüter C, Segers FH, Menezes C, Vollet-Neto A, Falcón T, von Zuben L et al (2017) Repeated evolution of soldier sub-castes suggests parasitism drives social complexity in stingless bees. Nat Commun 8:1–8
Grüter C (2020) Stingless bees. Springer International Publishing, Cham
Guevara DA, Gonzalez VH, Ospina R (2020) Stingless robber bees of the genus Lestrimelitta in Colombia (Hymenoptera: Apidae: Meliponini). Caldasia 42:17–29
Helanterä H, Ratnieks FLW (2008) Geometry explains the benefits of division of labour in a leafcutter ant. Porc. R Soc B Biol Sci 275:1255–1260
Hölldobler B, Engel H (1978) Tergal and sternal glands in ants. Psyche 85:285–330
Hölldobler B, Wilson EO et al (1990) The ants. Harvard University Press
Hölldobler, B., Wilson, E. O., and et al. (2009). The superorganism: the beauty, elegance, and strangeness of insect societies. WW Norton and Company
Hölldobler B, Plowes NJR, Johnson RA, Nishshanka U, Liu C, Attygalle AB (2013) Pygidial gland chemistry and potential alarm-recruitment function in column foraging, but not solitary, Nearctic Messor harvesting ants (Hymenoptera: Formicidae: Myrmicinae). J Insect Physiol 59:863–869
Jarau S, Hrncir M (2009) Food exploitation by social insects: ecological, behavioral, and theoretical approaches. CRC Press
Johnson LK (1987) The pyrrhic victory of nest-robbing bees: did they use the wrong pheromone? Biotropica. 19:188–189
Kendall LK, Rader R, Gagic V, Cariveau DP, Albrecht M, Baldock KC, Freitas BM, Hall M, Holzschuh A, Molina FP, Morten JM (2019) Pollinator size and its consequences: Robust estimates of body size in pollinating insects. Ecol Evol 1702–1714
Kerr WE (1951) Bases para o estudo da genética de populações dos Hymenoptera em geral e dos Apinae sociais em particular. Anais Escola Superior Agric Luiz Queiroz 8:219–354
Luzzio FA, Fitch RW, Moore WJ, Mudd KJ (1999) A facile oxidation of alcohols using pyridinium chlorochromate/silica gel. J Chem Educ 76:974
Mascena VM, Nogueira DS, Silva CM, Freitas BM (2017) First record of the stingless bee Lestrimelitta rufa (Friese) (Hymenoptera: Apidae: Meliponini) in NE Brazil and its cleptobiotic behavior. Sociobiology 64:359–362
McGregor P (2005) Animal communication networks. Cambridge University Press
Michener CD (2000) The bees of the world (Vol. 1). JHU Press
Moure JS, Nogueira-Neto P, Kerr WE (1958) Evolutionary problems among meliponinae (Hymenoptera, Apidae). Proceedings of the Xth Int. Congr. Entomology, Montreal, pp 481–493
Müller F (1874) The habits of various insects. Nature 10:102–103
Nogueira-Neto P (1970) Behavior problems related to the pillages made by some parasitic stingless bees (Meliponinae, Apidae). Aronson LR (ed) Development and evolution of behavior: essays in memory of TC Schneirla. W. H. Freeman, San Francisco, 416–434
Nunes TM, Von Zuben LG, Costa L, Venturieri GC (2014) Defensive repertoire of the stingless bee Melipona flavolineata Friese (Hymenoptera: Apidae). Sociobiology 61:541–546
Orlova M, Amsalem E (2019) Context matters: plasticity in response to pheromones regulating reproduction and collective behavior in social Hymenoptera. Curr Opin Insect Sci 35:69–76
Ottoboni MA (1984) The dose makes the poison. A plain-language guide to toxicology. In Vincente Books, 5
Papachristoforou A et al (2012) The bite of the honeybee: 2-heptanone secreted from honeybee mandibles during a bite acts as a local anaesthetic in insects and mammals. PLoS One 7(10):e47432
Pompeu MS, Silveira FA (2005) Reaction of Melipona rufiventris Lepeletier to citral and against an attack by the cleptobiotic bee Lestrimelitta limao (Smith) (Hymenoptera: Apidae: Meliponina). Braz J Biol 65:189–191
Quezada-Euán JJG, González-Acereto JA (2002) Notes on the nest habits and host range of cleptobiotic Lestrimelitta niitkib (Ayala 1999) (Hymenoptera: Meliponini) from the Yucatan Peninsula, Mexico. Acta Zool Mex 86:245–249
Quezada-Euán JJG, Ramírez J, Eltz T, Pokorny T, Medina R, Monsreal R (2013) Does sensory deception matter in eusocial obligate food robber systems? A study of Lestrimelitta and stingless bee hosts. Anim Behav 85:817–823
Rinderer TE, Blum MS, Fales HM, Bian Z, Jones TH, Buco SM, Lancaster VA, Danka RG, Howard DF (1988) Nest plundering allomones of the fire bee Trigona (Oxytrigona) mellicolor. J Chem Ecol 14:495–501
Roubik DW, Smith BH, Carlson RG (1987) Formic acid in caustic cephalic secretions of stingless bee, Oxytrigona (Hymenoptera: Apidae). J Chem Ecol 13:1079–1086
Roubik DW (1992) Ecology and natural history of tropical bees. Cambridge University Press
Ruther J, Meiners T, Steidle JLM (2002) Rich in phenomena-lacking in terms. A classification of kairomones. Chemoecology 2002(12):161–167
Sakagami SF, Laroca S (1963) Additional observations on the habits of the cleptobiotic stingless bees, the genus Lestrimelitta friese (Hymenoptera, Apoidea). 北海道大學理學部紀要 15:319–339
Sakagami SF, Roubik DW, Zucchi R (1993) Ethology of the robber stingless bee, Lestrimelitta limao (Hymenoptera: Apidae). Sociobiology. 21:237–277
Schorkopf DLP, Hrncir M, Mateus S, Zucchi R, Schmidt VM, Barth FG (2009) Mandibular gland secretions of meliponine worker bees: further evidence for their role in interspecific and intraspecific defence and aggression and against their role in food source signalling. J Exp Biol 212:1153–1162
Schwarz HF (1948) Stingless bees (Meliponidae) of the Western hemisphere. Lestrimelitta and the following subgenera of Trigona, Paratrigona, Swarziana, Parapartamona, Cephalotrigona, Oxytrigona, Scaura, and Mourella. Abejas Jicotes (Meliponidae) Del Hemisferio Occidental. Bull Am Mus Nat Hist 90:1–536
Segers FH, von Zuben L, Grüter C (2016) Local differences in parasitism and competition shape defensive investment in a polymorphic eusocial bee. Ecology 97:417–426
Shackleton K (2015) Suicidal biting in stingless bees. Bee World 92:21–23
Villalta I, Abril S, Cerdá X, Boulay R (2018) Queen control or queen signal in ants: what remains of the controversy 25 years after Keller and Nonacs’ seminal paper? J Chem Ecol 44:805–817
von Zuben LG, Nunes TM (2014) A scientific note on the presence of functional tibia for pollen transportation in the robber bee Lestrimelitta limao Smith (Hymenoptera: Apidae: Meliponini). Sociobiology 61:570–572
Von Zuben LG, Schorkopf DLP, Elias LG, Vaz ALL, Favaris AP, Clososki GC, Bento JMS, Nunes TM (2016) Interspecific chemical communication in raids of the robber bee Lestrimelitta limao. Insect Soc 63:339–347
Wittmann D (1985) Aerial defense of the nest by workers of the stingless bee Trigona (Tetragonisca) angustula (Latreille) (Hymenoptera: Apidae). Behav Ecol Sociobiol 16:111–114
Yamasaki T, Narahashi T (1959) The effects of potassium and sodium ions on the resting and action potentials of the cockroach giant axon. J Insect Physiol 3:146–158
Acknowledgements
This research was made possible through the efforts of multiple individuals, including Erik de Jesus Solórzano-Gordillo, Karen Santos, Danielle Nghiem, Alex Neskovic, Anna Dipaola, and Thomas Leung who have made the work possible. We would also like to thank Ralph Tyler Jack-McCollough for his preliminary work on the L. niitkib colonies. We would also like to thank the detailed comments and suggestions of the two anonymous reviewers who helped to significantly improve our manuscript.
Author information
Authors and Affiliations
Contributions
CCJ, DS, and JCN designed the experiments and wrote the manuscript. CCJ and JCN analyzed the data. DS provided bee colonies’ logistical support and conducted the SEM microscope experiment. LC analyzed the MGP and contributed key chemicals and reagents. CCJ provided financial support via the Scripps Institution of Oceanography. DS and JCN provided funding via UC Mexus grant CN13-620 and SEP-CONACYT Mexican National Council on Science and Technology Grant 128702.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by O. Rueppell
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
James, C.C., Sánchez, D., Cruz-López, L. et al. Fighting ability and the toxicity of raiding pheromone in an obligate kleptoparasite, the stingless bee Lestrimelitta niitkib. Behav Ecol Sociobiol 76, 38 (2022). https://doi.org/10.1007/s00265-022-03129-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03129-1