Abstract
Complex sexual signals spanning multiple sensory modalities may be common in nature, yet few studies have explored how combinations of phenotypic traits influence male attractiveness and mating success. Here, we investigate whether combinations of multiple male phenotypic traits (both within and across sensory modalities) predict male mating and fertilization success in the critically endangered southern corroboree frog, Pseudophryne corroboree. We conducted breeding trials in a standardized captive environment where females were given the opportunity to choose between multiple males over the duration of the breeding season. For each male, we measured multiple call traits, aspects of coloration, body size, and age. We found that complex interactions between multiple traits best predicted male mating and fertilization success. In general, males with lower call frequency, lower call rate, and shorter call duration had the highest mating and fertilization success. Fertilization success was additionally linked to male body size and age. These findings suggest that female P. corroboree select mates based on a suite of acoustic traits, adding to a growing body of evidence that females use multiple traits to assess male quality. Our results also suggest that females may combine information from multiple signals non-additively. Moreover, our results imply that females gain direct fertility benefits from their mate choice decisions. We argue that understanding female mate choice based on various signals across multiple sensory modalities has important implications for the integration of mate choice into conservation breeding programs and needs to be considered when developing behavior-based captive breeding strategies.
Significance statement
Sexual signals are often highly complex, yet we know little about how multiple signal components both within and across various sensory modalities predict male mating success. We investigated whether combinations of multiple phenotypic traits (within and across sensory modalities) predicted male breeding success in threatened corroboree frogs. We conducted captive breeding trials in a homogeneous environment, where females could choose between multiple males over the duration of a single breeding season. We found that interactions between multiple male traits predicted mating and fertilization success. Males with lower call frequency, call rate, and duration had higher mating success. Fertilization success was also linked to acoustic signals, body size, and age. Understanding mate choice for multiple traits further elucidates the complexity of female mate choice. This study is one of the first to consider the conservation implications of multimodal signaling in mate choice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Female mate choice is a strong evolutionary factor that can have significant consequences for female fitness (Andersson 1994; Rosenthal 2017). By preferentially mating with certain males, females can significantly improve their reproductive success, as well as the fitness of their offspring (Petrie 1994; Martin-Wintle et al. 2015; Hartnett et al. 2018; Parrott et al. 2019). In resource-based mating systems, where males provide resources essential for reproduction, females can obtain a combination of both direct and genetic benefits from their mating decisions, as these benefits are not mutually exclusive (Candolin 2003; Bussière et al. 2005). In these systems, females likely use multiple signals to evaluate the quality of potential mates and the probability of securing direct and/or genetic benefits (Candolin 2003). In a diversity of taxa, females discriminate among males based on various condition-dependent secondary sexual traits (such as extravagant courtship displays, ornamentation, or bright coloration) that can act as an honest signal of a male’s capacity to secure resources (Reaney and Backwell 2007), fertility (Robertson 1990), potential to provide paternal care (Montoya and Torres 2015), and/or genetic quality (Montoya and Torres 2015). Such benefits not only stand to elevate female fitness but may also improve the viability of populations (Cally et al. 2019).
Despite the significant fitness benefits that can derive from female mate choice, knowledge of intersexual selection is rarely incorporated into conservation breeding programs (CBPs, encompassing captive breeding and reintroduction) that aim to conserve and manage endangered species (Martin-Wintle et al. 2019). Many CBPs aim to establish viable, self-sustaining populations ex situ, while also providing genetically diverse individuals for population reintroduction in situ. However, for some threatened species, breeding success in captive colonies is unreliable (Wielebnowski 1998). Reproductive failures are often attributed to a lack of knowledge of the reproductive ecology of a target species, including mate choice behavior. Additionally, providing opportunities for mate choice may be disregarded in favor of genetic goals (i.e., individuals are strategically paired to maintain sufficient genetic variation). Yet, a growing body of empirical research has demonstrated that incorporating mate choice into CBPs can significantly bolster reproductive output and offspring fitness (Hartnett et al. 2018; Martin-Wintle et al. 2019), as well as preserve natural reproductive behavior and enhance animal welfare (e.g., by decreasing stress and aggression between mates) (Asa et al. 2011). Considering these benefits, gathering information on mechanisms of mate choice in species targeted for captive breeding has been identified as a crucial step towards integrating knowledge of sexual selection into CBPs (Quader 2005; Asa et al. 2011; Chargé et al. 2014). Specifically, advancing our understanding of factors that determine male attractiveness, and the phenotypic traits used by females to discriminate among potential mates, is likely to facilitate the incorporation of mate choice into captive management, and increase CBP outcomes (Asa et al. 2011; Martin-Wintle et al. 2019). Captive environments provide an ideal opportunity to dissect the mechanisms of female mate choice because mating contexts can be reliably manipulated to uncover the importance of different variables. For instance, breeding environments can be manipulated so that they are relatively homogeneous (compared with natural breeding environments), resulting in less variation in territory/resource quality, and/or climatic conditions (such as temperature and humidity), which can influence variation in male signaling. This enables biologists and managers to more easily examine how variation in male phenotype influences patterns of female mate choice and male mating success in captive breeding colonies.
Among amphibians globally, CBPs are increasingly being established to assist with the recovery of threatened species (Zippel et al. 2011), though there has been little attempt to use knowledge of mate choice to improve the captive breeding and reintroduction of endangered amphibians (but see Settle et al. 2018; Walls and Gabor 2019). This is surprising because for over 50 years, anuran amphibians have been a model group for the study of intersexual selection, and the mechanisms of female mate choice are extremely well understood (Wells 2007). In anuran amphibians, females evaluate potential mates based on multiple secondary sexual characteristics (Gerhardt and Huber 2002; Wells 2007). In particular, there are four main male traits that appear to provide honest signals of male quality: body size, age, call characteristics, and color (Sheldon et al. 2003; Forsman and Hagman 2006). In various anurans, male body size (which is often correlated with age) can predict male mating success, with evidence that females prefer larger and older males (Rausch et al. 2014; Kelleher et al. 2021a). Empirical evidence suggests that larger males may provide direct benefits in the form of higher fertilization success and/or genetic benefits by supplying offspring with good genes (Berven 1987; Robertson 1990; Rausch et al. 2014). Male age can also influence female mate choice in anurans independent of body size (Felton et al. 2006). Females might prefer older males as they have demonstrated their ability to survive, indicating superior genetic quality (Brooks and Kemp 2001). In most anuran species, males advertise to females by calling, and females select males based on a range of call traits, such as call peak frequency, pulse repetition rate, and duration (Gerhardt and Huber 2002). Calls are costly to produce (they are energetically expensive and increase predation and desiccation risk), so call traits can reflect male genetic quality (Gerhardt and Huber 2002). Indeed, there is evidence that by preferentially mating with males that produce longer or more complex calls, females can produce offspring that perform better during larval or juvenile life stages (Welch et al. 1998). Finally, visual traits (such as color) may also indicate male quality. Females have been shown to discriminate between potential mates based on numerous aspects of male coloration, including brightness (luminance), color saturation (chroma), and hue, and this is not necessarily restricted to species that exhibit obvious sexual dichromatism (Gomez et al. 2009; Maan and Cummings 2009; Dreher et al. 2017). Assuming that color pigments are costly to acquire and express, variation in color could also provide females with traits that reliably signal male quality (Gomez et al. 2009). Critically, however, sexual signals are often highly complex (Candolin 2003), and a growing number of studies have demonstrated that females use a combination of signal components (both within and across sensory modalities) to assess male quality (Burke and Murphy 2007; Dreher and Pröhl 2014). Based on these findings, mate choice studies should aim to take an integrated, multivariate approach, which considers the influence of multiple traits simultaneously (as opposed to a traditional single trait approach), and tests for interactive effects between various traits (Dreher and Pröhl 2014).
The southern corroboree frog, Pseudophryne corroboree, is a terrestrial breeding anuran and is one of Australia’s most critically endangered vertebrate species. Since the appearance of the deadly pathogen Batrachochytrium dendrobatidis (Bd, the amphibian chytrid fungus) in Australia in the 1970s, P. corroboree has experienced extreme population declines (Hunter et al. 2010), with less than 50 mature individuals currently remaining in the wild. The species is now the subject of a large-scale, multi-institutional CBP established in 2003 (McFadden et al. 2013). While protocols have been established that allow P. corroboree to be successfully bred in captivity, very little is currently known about factors influencing male mating success and patterns of mate choice. Female mate choice is apparent in captive breeding enclosures, where there is a distinct reproductive skew (approximately 30–50% of males receive successful matings) (McFadden et al. 2013). This skew indicates that some males are more attractive to females than others. Additionally, a proportion of females fail to breed annually if they do not encounter a suitable male (McFadden et al. 2013). These observations point towards female mate choice being an important component of the P. corroboree mating system. Male sexual coercion or male-male competition is not expected to strongly influence patterns of male mating success in P. corroboree because females move between the nests of multiple males before mating, and during these interactions males show no signs of attempting to force copulation (MSM, personal observation). Moreover, there is a distinct absence of male physical aggression, or other traits typically associated with intense intrasexual selection in anurans (e.g., nuptial spines, enlarged forearms, heads, or body size). In a recent field study in northern corroboree frogs (Pseudophryne pengilleyi), parentage analysis also provided limited evidence for alternative mating tactics such as satellite or sneaking behavior and the incidence of nest takeover was very low (Kelleher et al. 2021a). While P. corroboree males do have a distinct territorial call, these appear to function in establishing spacing between males at the time when choruses are first established, with no evidence that resident males suppress advertisement calling by their neighbors (suggestive of a dear enemy effect). A recent, preliminary investigation into female mate preferences in P. corroboree suggested that male dominant call frequency may be important in mate evaluation (Kelleher et al. 2021b); however, females may use a suite of different traits to assess potential mates. Advancing our understanding of factors that influence mate choice in P. corroboree may provide conservation managers with knowledge needed to develop behavior-based captive breeding strategies, which may maximize colony reproductive output and population viability.
The aim of the present study was to investigate patterns of male mating success in P. corroboree to inform the CBP for this species. Specifically, we investigated whether variation in multiple male phenotypic traits (call characteristics, coloration, age, body size) predicted: (1) male mating success and (2) male fertilization success. These aims were addressed by conducting breeding trials in a captive colony where male nest quality was standardized and females were able to choose between multiple potential mates.
Methods
Study species
The southern corroboree frog (P. corroboree) is a small (23–30 mm snout-vent length) terrestrial anuran from the family Myobatrachidae. The species is characterized by its striking bright yellow and black longitudinal stripes, located on its dorsal surface (Fig. 1). P. corroboree is endemic to Kosciuszko National Park in south-eastern Australia and its distribution is restricted to areas above 1300 m in elevation. P. corroboree females reach sexual maturity between 3 and 4 years old in captivity, and 4 and 5 years old in the wild, while males reach sexual maturity between 2 and 3 years old in captivity and 3 and 4 years old in the wild (Hunter 2000). P. corroboree breeds annually, with the breeding season commencing in mid to late summer (Osborne 1991). Male P. corroboree construct small, terrestrial nests (depressions) in sphagnum bogs or wet tussock grasslands, adjacent to ephemeral pools, or watercourses (Osborne 1991). Males call to attract females, with peak calling activity typically occurring from January to February (Pengilley 1971). Females oviposit within the nest chamber and then leave, while males remain with the eggs for several weeks (Osborne, 1991). Female clutch size ranges from 16 to 40 eggs (mean = 26.4) (Osborne 1991). Observations in nature and in captivity indicate that females can be monandrous and lay all their eggs in one nest (Pengilley 1973; McFadden et al. 2013). However, there is also evidence that some females may exhibit sequential polyandry and partition their clutch between the nests of multiple males (Pengilley 1973; McFadden et al. 2013). Of note, sequential polyandry is common in other closely related Pseudophryne species, such as Pseudophryne bibronii (Byrne and Keogh 2009). Fertilized eggs undergo intracapsular development inside the nest chamber, until they enter diapause, where development is suspended at Gosner stage 27 (Osborne 1991). Hatching is triggered by heavy autumn rain, which floods nest sites. Tadpoles are flushed into adjacent ephemeral pools or water bodies, where they continue to develop until they metamorphose in spring.
Study animals and husbandry
All frogs used in the present study were initially collected as eggs from extant wild populations between 2003 and 2012. After eggs were collected, they were transported to Taronga Zoo, NSW, Australia, where they were hatched and reared to adulthood in a biosecurity facility. Outside of the breeding season, all frogs were housed in same-sex groups (2–8 frogs) in clear, plastic enclosures (28 cm L × 17 cm W × 18 cm H). Each enclosure contained a base layer of fine aquarium gravel, covered by a layer of sphagnum moss (Brunnings, Australia). Frogs were fed Acheta domestica crickets (6–10 days old) twice weekly. Crickets were dusted with calcium powder before every feed (calcium with vit. D3, Rep-Cal Research Labs, USA), and a multivitamin supplement before every second feed (Herptivite, Rep-Cal Research Labs, USA). Enclosures were flushed with reverse-osmosis (R.O.) water twice weekly to prevent the accumulation of detritus and nitrogenous waste. Inside the facility, frogs were exposed to natural ambient light from a nearby window and provided with UV-enriched light supplied by a single Reptisun lamp (36″ 10.0 UV-B T8, Reptisun) suspended above the enclosures, approximately 36 cm from the frogs. UV lights were controlled by an external light sensitive switch (HPM, NSW, Australia) which detected changes in outside lighting conditions and simulated the natural local photoperiod. The facility was temperature-controlled, and ambient air temperature inside the facility was cycled annually, to reflect natural seasonal changes, which included a 6-week hibernation period. Temperature throughout the year ranged from 5 °C during the winter hibernation period, to a maximum of 20 °C during the breeding season. During the winter hibernation period, feeding ceased and resumed once the temperature was above 15 °C.
Captive breeding design
To determine whether male phenotypic traits predict breeding success in P. corroboree, captive breeding trials were conducted in eight glass breeding tanks (tank A, B, C, D, E, F, G, or H; 135 cm L × 55 cm H and 55 cm W, Supplementary Fig. 1) that were purposefully built for the captive breeding of P. corroboree at Taronga Zoo (McFadden et al. 2013). Each breeding tank was open at the top and contained a base layer of white aquarium gravel and a thick plant layer of live sphagnum moss (Supplementary Fig. 1). In nature, male corroboree frogs form choruses and cluster together in small groups (often around the edge of ephemeral pools or around small pieces of vegetation), so the size of the breeding area and proximity of male nest sites within the breeding tanks reflects natural conditions. Of note, the breeding tanks are suitable for assessing mate choice because P. corroboree call and breed deep within the live sphagnum moss, which limits the potential for sound to reverberate. Lighting to the breeding tanks was provided by one UV-enhanced lamp (36″ 10.0 UV-B T8, Reptisun) and one plant grow light bulb (F30W Gro-Lux T8, Sylvania), which were both controlled by an external light sensitive switch (HPM, NSW, Australia) which simulated the natural local photoperiod. During captive breeding trials (19 December 2018–15 March 2019), frogs were maintained on a 20 °C/17 °C day/night temperature cycle. The breeding tanks were hydrated by an internal automated misting system. Reverse-osmosis filtered water was delivered once per day for one minute through three spray nozzles situated above each tank (below the tank lid). This watering system ensured that the live sphagnum moss was 100% saturated at all times (confirmed via measurements with a moisture probe meter; MPM-160-B, 12-bit resolution, ICT International Pty Ltd, Armidale, Australia). This design ensured that the nesting environments were homogeneous across all breeding tanks.
In each captive breeding trial (N = 8, one trial per breeding tank), females were given a choice between multiple males simultaneously for the duration of the breeding season (i.e., live males were present for the entirety of the breeding season). For the purpose of this study, to allow for considerable variation among males (and potential for female discrimination), eight males and eight females were placed in each breeding tank. This resulted in each female being able to choose between a maximum of eight different males in a breeding chorus, which simulated a natural breeding chorus, where males aggregate in small groups and acoustically interact. Males and females grouped together remained in their designated breeding tanks for the duration of the breeding season. While the operational sex ratio is not known for wild P. corroboree, observations indicate that females remain in the chorus for an extended period assessing males before mating (D. Hunter, personal communication). Additionally, female arrival to breeding sites in Pseudophryne species is controlled by a combination of climatic and social conditions and reasonably synchronized (O’Brien et al. 2021), so there are typically groups of females assessing groups of males at the same time. Moreover, this design follows the current P. corroboree captive breeding protocol where groups of multiple females (N = 2–8) and multiple males (N = 2–8) are assigned to breeding tanks based on a maximum avoidance of inbreeding genetic breeding scheme (OEH 2012). For this study instead of sorting individuals based on genetics, males were sorted into breeding tanks based on their pre-breeding body weight (weight as of the 19th of November, range = 1.46–2.65 g, mean = 2.03 g), as male mating and fertilization success is related to body size in various anuran species, and is often linked to male call characteristics (Wells 2007). Males were weighed and then ordered from largest to smallest. The eight largest males were then randomized into each of the eight tanks (tank A, B, C, D, E, F, G, or H). Then, the eight smallest males were randomized into each of the eight tanks. This process continued until all 64 males had been assigned to breeding tanks and ensured that small, medium, and large size males were present in every tank. The mean body size of males did not differ between breeding tanks (ANOVA: F7,56 = 0.0919, p = 0.9986, Supplementary Fig. 2), nor did variance in body weight (Levene’s test: F7,56 = 0.1749, p = 0.9894, Supplementary Fig. 2). Visibly gravid females were also assigned randomly to tanks via pre-breeding body weight (weight as of the 19th of November 2018; range = 2.74–4.44 g, mean = 3.37 g). The mean body size of females did not differ between breeding tanks (ANOVA: F7,56 = 0.1106, p = 0.9974, Supplementary Fig. 3) nor did variance (Levene’s test: F7,56 = 0.0594, p = 0.9997, Supplementary Fig. 3). At the time of the study, males ranged in age from 6 to 15 years old post metamorphosis, and females ranged in age from 6 to 14 years old post metamorphosis (known from husbandry records based on when eggs were collected from the wild).
Data collection
Captive breeding trials commenced at the beginning of the breeding season, 19 December 2018, when males started to advertise to females by calling. Males (N = 64) were removed from same-sex housing and transferred into their designated breeding tanks (tanks A–H). Males were placed into the breeding tanks 6 weeks earlier than females. This was done to allow males to establish individual nest sites (as they would do in the wild) within the tanks before introducing females and is consistent with the current P. corroboree captive breeding protocol (McFadden et al. 2013). Immediately prior to being placed into the tank, each male was weighed using a portable balance, and snout-vent length (SVL) was measured with calipers. Next, each male was digitally photographed to enable quantification of their yellow dorsal coloration at a later date (see the “Male color analysis” section). Frogs were photographed inside a custom-built, portable photography light arena. The arena consisted of two opaque cylindrical containers stacked on top of each other, with integrated white LED strip lights attached around the inside walls of the arena. This prevented shadowing and created a standardized lighting environment for all photos. Photographs were taken with a Canon EOS 70D camera with a standard lens (Canon EFS 18–55 mm lens) that was positioned on top of the arena, with the camera lens placed through a viewing hole at the top of the arena. The distance between the camera lens and the frog was 28 cm. Photographs were taken in raw format with the following settings: ISO = 400, f = 11, shutter speed = 1/200. All color photographs included an X-rite ColorChecker Passport (X-rite, USA), which consists of 24 colored and grayscale squares against which the colors in each photo are standardized.
On the 4th of January 2019, approximately 2 weeks after males started actively calling, occupied male nest sites were identified and flagged on the surface of the moss (male nests are below the surface of the moss) using a small, white plastic disc marked with an individual nest ID number. Nest sites were flagged again on the 14th, 17th, and 23rd of January 2019, and call recordings started on the 29th of January 2019, once the majority of nests had been identified and peak calling activity was observed. Call recordings were taken three times a week during periods of high calling activity (07:00 h and 14:00 h) for the duration of the study. All males that were consistently calling during the recording periods were recorded using a Marantz Professional handheld solid-state recorder (Model No. PMD661MKII, D&M Holdings Inc. Tokyo, Japan) connected to a directional microphone (NTG2, Rode Microphones, Sydney, Australia) which was held approximately 30 cm above the focal male. Calls were recorded in .wav format at a sampling rate of 44.1 kHz with a 16-bit resolution.
Females (N = 64) were placed into their designated breeding tanks on January 31, 2019. To determine male mating success, all male nest sites were checked once a week on a Friday for six consecutive weeks (the entire breeding season). The first nest checks occurred 1 week after females first entered the breeding tanks (8 February 2019), with subsequent checks made on the following dates: February 15, February 22, March 1, March 8, and March 15, 2019. During each nest check, we identified the resident male (i.e., the male calling from the flagged nest), using their unique dorsal and ventral patterns, and recorded whether eggs were present or absent, and counted total egg number. When eggs were present, they were removed from the male’s nest and transferred into plastic containers on sphagnum moss. Male mating success was recorded as mated (yes/no) and the total number of eggs received over the entire breeding season (6-week period). We used the total number of eggs a male received as a measure of mating success because it was not possible to ascertain the total number of matings (females may partition their clutch among multiple nests). However, a higher egg number can be assumed to indicate a greater number of matings. This approach follows that of a recent field study in P. corroboree sister species, the northern corroboree frog, Pseudophryne pengilleyi (Kelleher et al. 2021a). After the breeding season was complete, on the 20th of March 2019, all eggs were assessed to determine whether they were fertile, based on whether there was evidence of embryo development. We then calculated the proportion of fertilized eggs for each male by dividing the number of fertilized eggs by the total number of eggs received. In regard to parentage, a recent field study in P. corroboree sister species, P. pengilleyi, found that a resident male is typically the genetic sire of all the eggs within their nest (Kelleher et al. 2021a), so we have assumed that is the case in this study. Of note, it was not possible to record data blind because this study required knowledge of individual male identity.
Male call analysis
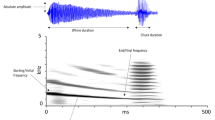
While advertising to females, P. corroboree males produce two main calls: a two-part call and a one-part call (Pengilley 1971; Osborne 1991). Oscillograms and spectrograms of both call types are presented in Fig. 2. The two-part call is comprised of two distinct components: a longer initial component, typically followed by a clear interphase, and then a shorter, pulsatile second component (Fig. 2; Pengilley 1971). Call recordings were analyzed using the sound analysis software Raven Pro v 1.5 (Cornell Lab of Ornithology, Bioacoustics Research Program, 2014). Temporal call traits (call duration, pulse repetition rate, and call rate) were obtained from oscillograms. Frequency information was obtained using Fast Fourier transformation, with a Blackman window function (window size = 1024 samples, 3-db bandwidth = 70.7 Hz, DFT = 2048 samples, grid spacing = 21.5 Hz, with 50% overlap) (following Kelleher et al. 2021a). For each male, we measured call traits (duration, frequency, and pulse repetition rate) for each component of the two-part call (1st and 2nd component, Fig. 2) and the one-part call (Fig. 2). For both the two-part and one-part call, multiple individual calls were analyzed (85% of males had between 5 and 12 calls analyzed for each call type), over 1 and 3 call recordings taken throughout the breeding season. This level of sampling is comparable to other studies investigating the effect of male call traits on mating success in frogs (e.g., Pröhl 2003; Smith and Roberts 2003b; Felton et al. 2006; Kelleher et al. 2021a). Average call traits for each call component were calculated as follows: average call duration (length from the beginning of the first pulse to the end of the last pulse of the call, seconds), average pulse repetition rate (number of pulses in the call divided by the call duration, pulses/sec), and average call peak frequency (frequency occurring at the highest amplitude, Hz). Call rate was calculated as the total number of calls (combined total number of two-part and one-part calls, ranging from 5 to 32 calls) given per minute from a male’s longest, continuous call recording (ranging from 30 s to 4 min). This resulted in ten call variables measured for each male: average duration of the 1st and 2nd components of the two-part call, and the one-part call, average frequency of the 1st and 2nd components of the two-part call, and one-part call, average pulse rate of the 1st and 2nd components of the two-part call, and one-part call and call rate. Of note, a small number of males (N = 7) had their average call duration, frequency, and pulse rate calculated from less than 5 calls for one call type (i.e., for either the two-part or one-part call). Although this level of sampling is comparable to other studies (Smith and Roberts 2003b; Kelleher et al. 2021a), the statistical analysis (outlined below in the “Statistical analysis” section) was run with these males excluded to ensure they were not driving patterns observed. As this was not the case, these males were retained to increase power of the analysis.
Male color analysis
Male P. corroboree color analysis followed methods described in Kelleher et al. (2021a Appendix). In brief, we obtained average red (R), blue (B), and green (G) pixel values from the yellow stripes on the entire dorsal surface (head, body, and legs) of each individual male using a custom Matlab script (Mathworks Inc., Natick, MA, USA) written by JAE. After standardized RGB values were calculated from the color standards in each digital photo, a second custom Matlab script written by JAE was used to determine a color space based upon calculations of hue, chroma, and luminance (detailed descriptions of hue, chroma, and luminance calculations are described in Cadena et al. 2017, 2018). Hue represents the color, measured as the angle relative to the axis (Endler 1990; Cadena et al. 2018), chroma is the “saturation” of the coloration and is measured as the distance from the origin (Endler 1990; Cadena et al. 2018), and luminance represents the brightness of the coloration and was calculated as the sum of the standardized RGB values (Endler 1990; Cadena et al. 2018). We did not use an eye model, because data on cone absorption spectra are not available for this species and family. In any case, our question here is simply whether these crude measures of color predict mate choice.
Statistical analysis
To investigate the influence of male phenotypic traits on male mating and fertilization success in P. corroboree, we measured 16 explanatory variables; (1) body weight, (2) age, (3) snout-vent length (SVL), (4) luminance, (5) hue, (6) chroma, (7) call rate, (8) average duration of two-part call, 1st component, (9) average duration of two-part call, 2nd component, (10) average duration of one-part call, (11) average frequency of two-part call, 1st component, (12) average frequency of two-part call, 2nd component, (13) average frequency of one-part call, (14) average pulse rate of two-part call 1st component, (15) average pulse rate of two-part call, 2nd component, and (16) average pulse rate of one-part call. Of the 64 males included in this study, 47 males were included in the statistical analysis. Twelve males were excluded as they failed to advertise to females, and an additional five males (four from tank A and one from tank B) were excluded during call analysis due to overlap on call recordings with neighboring males (we were unable to determine which call belonged to a focal male). Of note, four females died of unknown causes before laying eggs (two from tank D and two from tank H).
Prior to analysis, we checked for collinearity among all explanatory variables using a Pearson’s correlations matrix (see Supplementary Fig. 4). Variables with a significant correlation coefficient ≥ 0.5 were considered to be highly correlated (Zuur et al. 2009) so one of the two affected variables was removed from further analysis. Male age and hue were negatively correlated (r = − 0.50, p = 0.0004) and chroma and luminance were positively correlated (r = 0.79, p = < 0.0001), so hue and chroma were removed from further analysis. The average frequency of all calls were highly correlated (average frequency 1st component–2nd component: r = 0.85, p = < 0.001; average frequency 1st component–one-part call: r = 0.87, p = < 0.001; and average frequency 2nd component–one-part call: r = 0.87, p = < 0.001), so one measure of frequency (average frequency one-part call) was taken forward in the analysis. Finally, the average pulse rate of the 1st component and average pulse rate of the one-part call were positively correlated (r = 0.77, p = < 0.001) so the average pulse rate of the 1st component was removed from the analysis. Independent variables taken forward in the analysis were selected based on important predictor variables in a recent study investigating predictors of male mating success in P. corroboree sister species, P. pengilleyi (Kelleher et al. 2021a).
We used linear models to examine the potential effect of male body weight, SVL, and age on male call characteristics: call rate, call duration (all call components), call frequency (one-part call), and pulse rate of the 2nd component and of the one-part call. Before fitting models, all explanatory variables were first standardized to have a mean of zero and a standard deviation of 1 as variables were measured on vastly different scales (Zuur et al. 2009). This improves model convergence and calculates coefficient estimates on the same scale (Zuur et al. 2009). Linear models were fit for each call variable separately, with standardized body weight, SVL, and age entered as continuous explanatory variables. Call rate, average pulse rate, and average frequency of the one-part call were all log-transformed to improve normality.
To investigate predictors of male mating success we used generalized linear mixed-effects models (GLMM) with a zero altered negative binomial (ZANB) distribution and log link function. The ZANB GLMM models the data in two parts: (1) a binomial logistic regression with male mating success (yes or no) as the response variable and (2) a negative binomial GLMM of the count data, with the total number of eggs as the response variable (excluding zeros) (Zuur et al. 2009). As the data were over dispersed and zero-inflated, a ZANB distribution was used in place of a Poisson or standard negative binomial distribution. Our sample size (N = 47) limited the number of explanatory variables that could be fitted in the same model, so we used a two-step model selection process (following Kelleher et al. 2021a). First, we constructed 11 separate ZANB models each with the number of eggs as the response variable and one single explanatory variable as a predictor (see Supplementary Table 3). All standardized explanatory variables were entered into models as continuous predictors, and breeding tanks (A–H) were included in every model (in both the count and binomial part of the model) as a random effect. We then used an information-theoretic model selection process to rank the 11 single variable ZANB models based on their Akaike’s information criterion value with a correction for a small sample size (AICc). We compared these 11 separate models based on their AICc value and examined the model coefficients to determine whether the variable in question was important in the binomial or count part (or both) of the ZANB model. Variables that were within delta AICc < 2 of the top-ranked single variable model were retained for further analysis, these were as follows: age, call rate, luminance, all call durations, and average call frequency (see Supplementary Table 3). Next, we constructed 41 candidate ZANB models (including an intercept-only model) using these variables and ranked them based on their AICc value (see Supplementary Table 4 for a full list of candidate models). Due to our sample size, only interactions with call rate were considered, as call rate is often an important predictor of male mating success in anurans (Pröhl 2003; Wells 2007) and no model was constructed with complexity exceeding the limits of the sample size. The model with the lowest AICc value was considered to provide the best fit but models within delta AICc < 2 were considered to be plausible alternatives (Zuur et al. 2009; Symonds and Moussalli 2011). When multiple candidate models were within delta AICc < 2 of the top-ranked model, estimated coefficients and p-values were derived from model averaging (Zuur et al. 2009).
To determine predictors of male fertilization success, we used GLMMs with a binomial distribution and logit link function (following methods outlined in Zuur et al. 2009). For males that achieved mating success (N = 31), the proportion of fertilized eggs was entered as a response variable, with the total number of eggs overall entered as a model weight (Zuur et al. 2009) and breeding tanks (A–H) entered as a random effect. The model selection followed the same process described above. After modeling each explanatory variable separately, we retained the top six variables (based on their AICc value), plus one measure of body size (SVL) for further investigation (see Supplementary Table 5). These variables were as follows: age, call rate, average pulse rate (2nd component), average duration (1st and 2nd component), SVL, and average frequency. As for mating success, we then constructed 41 candidate models (including an intercept-only model, see Supplementary Table 6) and ranked them according to their AICc value. The model with the lowest AICc value was considered to provide the best fit, but models within delta AICc < 2 were also considered as alternative models (Zuur et al. 2009). Predictions were calculated for statistically significant predictors from the averaged model. We investigated model fit by visually examining diagnostic plots of residual versus fitted values for each predictor variable in the top-ranked model(s). As a supplementary analysis, we also investigated predictors of the total number of fertilized eggs using a GLMM with a negative binomial distribution and a log link function. We followed the same model selection process as described above and the analysis showed similar results to the proportion analysis, both for the top-ranked predictor variables and candidate models (see Supplementary Tables 7 and 8). All statistical analyses were completed in R version 3.5.1 (R Development Core Team 2018). GLMM analyses were conducted using the glmmTMB package (Brooks et al. 2017), while AICc and model averaging were conducted using the MuMIn package (Bartoń 2019).
Results
Variation in aspects of male phenotype
Of the 64 males included in the captive breeding trials, 81% (52/64) established individual nest sites and actively advertised to females by calling. The remaining 19% of males (12/64) failed to establish nest sites and were excluded from further analysis. Five advertising males were also excluded from further analysis due to call overlap with neighboring males (see Male call analysis and Statistical analysis sections). Thus, a total of 47 males were included in the statistical analysis.
Of the males included in the analysis (N = 47), body weight ranged from 1.61 to 2.80 g, with a mean (± SEM) of 2.23 g (± 0.04). Male SVL ranged from 23.5 to 27.8 mm, with a mean (± SEM) of 26.34 mm (± 0.14). There was a significant, positive correlation between male body weight and SVL (r = 0.38, p = 0.008, Supplementary Fig. 4). Male age ranged from 6 to 14 years, with a mean (± SEM) of 9.70 years (± 0.38). There was no significant correlation between male body weight or SVL and male age (Supplementary Fig. 4). Variation in male call and color traits are summarized in Supplementary Table 1. There was a significant positive relationship between SVL and male call rate; larger males had higher call rates (Supplementary Table 2). Additionally, there was a significant positive relationship between male body weight and average pulse rate of the one-part call; larger males had higher pulse repetition rates (Supplementary Table 2). Body weight, age, and SVL did not influence any of the other male call characteristics (see Supplementary Table 2).
Variation in male mating and fertilization success
Of the 64 males included in the captive breeding trials, 53% (34/64) of males gained matings (number of eggs > 0). Of the males included in the analyses (N = 47), 65% (31/47) of males received matings (number of eggs > 0). The total number of eggs in a male’s nest over the entire breeding season ranged from 3 to 95 eggs (Fig. 3), with an average of 28.6 eggs per nest. Of the males that achieved mating success (N = 31), fertilization rates ranged from 0 to 91.3% with an average fertilization rate of 48.6%.
Predictors of male mating success
Mating probability
There were 12 competing models (AICc < 2) to explain the probability of a male mating (Supplementary Table 4). These models contained different additive combinations of male age, luminance, and average call duration (of the 1st and 2nd component) as predictor variables (Supplementary Table 4). Male age, luminance, and average duration (1st component) appeared to have a positive effect on mating probability (Table 1) and average duration (2nd component) appeared to have a negative effect on mating probability (Table 1). However, there was no significant effect of any of these predictors on mating probability (Table 1).
Mating success (total number of eggs)
The best model to explain variation in the total number of eggs in a male’s nest contained the interaction between call rate and average call frequency of the one-part call (Supplementary Table 4). There was one competing model within 2 AICc points of this model, which contained the interaction between call rate and average call duration (Supplementary Table 4). There was a significant effect of both interactions on the number of eggs in a male’s nest (Table 1).
The effect of call duration (one-part call) on male mating success depended on call rate. Males with short calls and a low call rate had the highest numbers of eggs (Fig. 4a). When call rate was high, males with longer calls had higher egg numbers than males with shorter calls (Fig. 4a).
The effect of a the interaction between call rate and average call duration (one-part call) (seconds) and b the interaction between call rate and average call frequency (Hz) on male P. corroboree mating success (number of eggs > 0). Lines are model predictions with 95% confidence intervals. Note: Relationships presented between call duration, frequency, and call rate have been presented using a categorical interpretation of call rate for visualization purposes. These categories were calculated using the 25th, 50th, and 75th percentiles. Call rate values outside of this range are likely to present more extreme relationships than the ones presented here
The effect of call frequency on male mating success also depended on call rate. Overall, males with lower call frequencies had higher numbers of eggs than males with higher call frequencies (Fig. 4b). When call frequency was low, call rate did not significantly influence mating success (Fig. 4b). But, when call frequency was high, males with lower call rates had higher mating success than males with higher call rates.
Predictors of fertilization success
The best model to explain variation in male fertilization success (proportion of fertilized eggs) contained male age and the interaction between call rate and average call frequency as predictor variables (Supplementary Table 6). There was one competing model within 2 AICc points of this model, which contained average call frequency and the interaction between call rate and SVL as predictor variables (Supplementary Table 6).
There was a significant effect of male age on male fertilization success; older males had a higher proportion of fertilized eggs (Table 2 and Fig. 5). There was a significant decrease in the proportion of fertilized eggs with increasing call frequency, but the strength of this relationship depended on a male’s call rate (Fig. 6a). For males with lower call frequencies, fertilization success did not vary with call rate (Fig. 6a). But, for males with higher call frequencies, fertilization success varied with call rate; males with lower call rates had higher fertilization success than males with higher call rates.
The effect of the interaction between a male call rate and average call frequency (Hz) and b male call rate and SVL (mm) on male P. corroboree fertilization success (proportion of fertilized eggs). Lines are model predictions with 95% confidence intervals. In line with Fig. 4, call rate has been categorized (calculated using the 25th, 50th, and 75th percentiles) in order to visualize the interaction with frequency and SVL
The effect of body size (SVL) on fertilization success also depended on a male’s call rate. Small males had a high proportion of fertilized eggs irrespective of call rate (Fig. 6b). Large males with low call rates had higher fertilization success than large males with higher call rates (Fig. 6b).
Discussion
Understanding factors that influence female mate choice can potentially aid in the captive breeding of threatened species. Here, we investigated whether variation in male body size, age, calling behavior, and coloration predicted male reproductive outcomes in critically endangered P. corroboree. Our results revealed that both male mating and fertilization success were predicted by a complex interplay between multiple phenotypic traits. In particular, variation in male mating success (total egg number received) was strongly explained by a combination of interactions between male call rate, call duration, and call frequency. Fertilization success was also associated with call rate and call frequency, in addition to male body size and age. These findings suggest that females are using multiple traits (call traits and physical traits) to assess male quality and fertility and using them in different combinations. More broadly, these results add to a large body of empirical evidence that female mate choice decisions are based on information gained from multiple male traits (Candolin 2003; Starnberger et al. 2014; Mitoyen et al. 2019). Moreover, these results highlight that studies that only consider the influence of a single trait may fail to capture to complexity of mate choice (Taylor et al. 2007; Gould and Augustine 2020).
Effect of calling behavior
Males with low-frequency calls had higher mating and fertilization success than males with high-frequency calls (irrespective of call rate), a pattern that is relatively common in anurans (Wells 2007; Lesbarrères et al. 2008). This outcome suggests that P. corroboree males with low-frequency calls are highly attractive and is in line with the results of our recent manipulative phonotaxis study, which found that P. corroboree females significantly preferred low-frequency male advertisement calls (Kelleher et al. 2021b). Our findings strongly suggest that call frequency is an honest signal of male fertility and fertilization potential, as males with low call frequencies also had high fertilization success. This finding implies that female P. corroboree may preferentially select males with low call frequencies to receive direct fertility benefits. This finding is particularly noteworthy, as a direct relationship between male call frequency and fertilization success has rarely been reported in anurans. One example comes from a study on a wild population of the smooth toadlet (Uperoleia laevigata), where female mate choice based on male call frequency (which is related to male body size) also resulted in enhanced fertilization success (Robertson 1990). Interestingly, very few studies have investigated relationships between anuran male call traits and fertility, but there is evidence for such associations in birds and insects (Wagner and Harper 2003; Simmons et al. 2010). Therefore, the potential for females to assess male fertility based on acoustic signals may be more widespread than currently appreciated and requires further examination. Evidence to date has illustrated that fertility is generally related to visual signals (e.g., Lifjeld et al. 2011), but it is possible that acoustic signals may also provide strong and reliable indicators of male fertilization potential.
Calling behavior was also related to male mating and fertilization success in three other key and interactive ways: (1) males with less attractive, higher frequency calls had higher breeding success if they called at a lower rate, (2) males with short calls had higher mating success than males with longer calls, and males with short calls had the highest mating success if they called at a lower rate, and (3) call rate had little effect on mating success if calls were longer. These relationships were surprising as they contrast with past studies in anurans, which have reported that (1) females typically prefer males that invest more in calling (e.g., have longer calls or call at a higher rate) (Gerhardt and Huber 2002; Wells 2007) and (2) males with unattractive calls (e.g., high call frequency or shorter calls) can increase their relative attractiveness by increasing their calling effort (e.g., by calling at a higher rate or over more nights) (Morris and Yoon 1989; Smith and Roberts 2003b). In regard to call rate, we found that there was a positive association between call rate and body size (larger males had higher call rates), and large males with high call rates had very low fertilization success (see the “Effect of age and body size” section). This may explain why males with high call rates generally had lower success (and a high call rate did not improve the attractiveness of high frequency or short calls), as females may have been avoiding larger males due to the costs associated with low fertility (discussed below). Interestingly, however, call rate had little effect on mating success when call duration increased. There is no obvious explanation for this association. However, evidence from studies in other anurans, as well as birds and insects, suggest that females are often particularly sensitive to calls that contain a greater amount of energy (i.e., longer, faster rate, louder), possibly due to a pre-existing bias in the sensory system (Ryan and Keddy-Hector 1992). For example, longer calls have been shown to elicit a heightened stimulatory response from females (Ryan and Keddy-Hector 1992), and this stimulation can supersede the effect of other call traits (such as call rate). Moreover, there is also evidence that this increased stimulation can modify or reverse preferences for other traits (Klump and Gerhardt 1987; Ryan and Keddy-Hector 1992). Additionally, these relationships might exhibit a high level of complexity because anuran mating behavior is often plastic. For instance, there is often considerable among and within-individual variation in male calling behavior, and males can use different calling strategies depending on social context (Byrne 2008) such as the strategies being used by competitors (Lesbarrères and Lodé 2002). Additionally, males can also adopt different calling strategies depending on body size or age (Smith and Roberts 2003b). Female anurans can also exhibit reversible phenotypic plasticity in mate preferences, and mate choice can be influenced by a multitude of factors such as female age, reproductive state, prior social experience, and social interactions between chorusing males (such as decoy effects) (Jennions and Petrie 1997; Ryan et al. 2019).
Collectively, our findings suggest that female P. corroboree combine information from multiple acoustic traits non-additively and that the strength of preference for one trait has the potential to be altered by the strength of other traits (Candolin 2003; Smith and Roberts 2003a). Similar results have been reported for other anurans (Smith and Roberts 2003a; Burke and Murphy 2007; Castellano and Rosso 2007). For example, phonotaxis trials in gray treefrogs (Hyla versicolor) have shown that females appear to weigh call duration more heavily than call rate and that they discriminate between males in favor of long calls at a slow rate over short calls at a higher rate (Gerhardt 2001). In the barking tree frog (Hyla gratiosa), females also combine signal components non-additively, and preferences for call duration and call rate vary depending on the percentage difference between the two stimuli (Burke and Murphy 2007). It is possible that female P. corroboree may weigh call rate more heavily when assessing males with higher call frequency or shorter calls, as opposed to when call frequency is low (which is likely highly attractive and an indicator of male quality) or when call duration is long (as this may act as a hyper stimulus). It is important to consider that patterns of female mate choice observed here could also reflect among-individual variation in female preferences for particular traits (e.g., some females prefer high call frequency while others prefer low frequency) (Jennions et al. 1995); however, our recent manipulative phonotaxis study in P. corroboree did not provide any evidence for among-individual variation in preferences for call frequency (Kelleher et al. 2021b). Female mate choice decisions could also reflect variation in how females’ weight multiple male attributes (e.g., females assess and combine information from multiple traits but some females weight call rate more heavily while others weight duration more heavily) (Castellano and Rosso 2007), or variation in investment strategies (e.g., level of investment in mate sampling) (Jennions and Petrie 1997; Rosenthal 2017). Clearly, mate choice in P. corroboree is complex, and more experimental work is needed to determine exactly how female P. corroboree use and rank different components of acoustic signals, especially when assessing multiple males simultaneously within a dynamic breeding chorus.
Effect of color
We found no evidence that aspects of male dorsal coloration (hue, chroma, or luminance) influenced male mating or fertilization success in our captive population. This finding aligns with the results of our recent field study investigating mate choice in the closely related sister species, P. pengilleyi, which also revealed that male dorsal coloration did not predict mating success (Kelleher et al. 2021a). The most plausible explanation for this finding is that coloration in corroboree frogs has an aposematic function, as opposed to the color being a possible sexual signal that indicates male quality (such as fertility). This reasoning is supported by the distinct absence of noticeable dichromatism in P. corroboree and the fact that both sexes contain toxic alkaloid compounds in their skin (Daly et al. 2005). Interestingly, we found evidence that male hue was related to male age, with older frogs exhibiting a more orange coloration. This relationship may be due to older frogs having increased access to food resources over their lifetime (compared to younger frogs), and subsequently a greater amount of time to acquire particular nutrients that may affect integument (skin) coloration (Umbers et al. 2016; Brenes-Soto et al. 2017; Walton et al. 2021). One group of dietary nutrients known to affect yellow/orange coloration are carotenoids (Brenes-Soto et al. 2017). Dietary carotenoids are present in captive corroboree diets and recent experimental work has shown that P. corroboree possesses mechanisms needed to sequester and utilize dietary carotenoids (Umbers et al. 2016; McInerney et al. 2019). Moreover, enhanced carotenoid intake has been shown to alter skin coloration from a yellow to more orange coloration (Umbers et al. 2016). Thus, although the primary function of bright coloration in P. corroboree is likely to be predator defense, color may also indirectly signal male age or nutritional status (Brenes-Soto et al. 2017). Therefore, the possibility remains that females may use color variation in their mating decisions.
Effect of age and body size
We found no relationship between male body size and age in our study population, yet our results revealed that both these traits independently predicted male fertilization success. Older P. corroboree males had significantly higher fertilization success than younger males. There are several possible explanations for this finding. One possibility is that older males have greater fertilization potential (functional fertility) because they have higher sperm production or superior quality sperm (Sheldon 1994; Brooks and Kemp 2001). Although the relationship between functional fertility and age is yet to be explicitly examined in anurans, studies in birds, fish, and invertebrates have shown that older males often possess a greater fertilization capacity, and that by selecting older males as mates females can insure against functional infertility (Schmoll et al. 2007). An alternative explanation is that older males have higher fertilization success because they are of higher genetic quality (Brooks and Kemp 2001). Viability indicator models of sexual selection predict that male age may reliably indicate intrinsic genetic quality and that older males signal their viability via their proven ability to survive (Brooks and Kemp 2001). Critically, intrinsic genetic quality may also be associated with superior sperm performance (Locatello et al. 2006), with evidence in various taxa that males of higher genetic quality have increased sperm viability, motility, and longevity (Locatello et al. 2006; Evans et al. 2007); these are sperm traits known to influence fertilization success. Obtaining direct fertility benefits from mate choice is likely to be particularly important to females in polygynous mating systems, where males mate consecutively with multiple females, and highly successful males risk becoming sperm depleted (Scarponi and Godin 2018). In the P. corroboree mating system males are polygynous (Kelleher et al. 2021a), and in the present study the most successful males would have mated with at least three females (estimated based on female average clutch size and the total number of eggs they received throughout the entire season). Additionally, P. corroboree males have a small relative testes size compared with other myobatrachid frogs (Byrne et al. 2002), so highly successful males may be at a particularly high risk of becoming sperm depleted. Accordingly, there may be strong selection on P. corroboree females to assess and choose males based on fertility (Scarponi and Godin 2018).
The relationship between body size and fertilization success was more complex. We found that the effect of body size on fertilization success depended on a male’s call rate, and, additionally, call rate was positively associated with male body size (larger males called at higher rates). Smaller males generally had higher fertilization success than larger males, regardless of call rate. Large males with high call rates had very low fertilization success. This finding was unexpected because larger males (that often invest more in sexual displays as they have greater energy reserves) are often reported to have high fertilization success, and females typically prefer large males as they provide direct fertility benefits (Pujolar et al. 2012). Conversely, our findings are in line with numerous studies demonstrating that smaller males may also have enhanced fertilization success (Klaus et al. 2011; Young et al. 2013). Smaller males may have higher fertilization success because they preferentially invest in sperm production, at the expense of investing in growth or elaborate sexual signals (Stearns 1989; Simmons et al. 2017; Lüpold et al. 2019). Such life-history tradeoffs have been reported in various taxa, with strong negative relationships reported between male body size, sexual signaling intensity, and fertility (Evans 2010; Sherman et al. 2010). For instance, in the quacking frog (Crinia georgiana), there is a negative relationship between male body size and sperm viability (proportion of live sperm), suggesting that smaller males allocate more resources to sperm production (Dziminski et al. 2010). Similarly, in Chinook salmon (Oncorhynchus tshawytscha), smaller subordinate males have higher sperm velocity and higher fertilization success than larger, dominant, ornamented males (Flannery et al. 2013; Young et al. 2013). It is possible that P. corroboree males also preferentially and strategically invest in certain fitness-determining traits over their lifetime. Corroboree frogs are long-lived; individuals have been reported to live for up to 9 years post metamorphosis in the wild (Hunter 2000), and 20 years post metamorphosis in captivity (MSM personal observation). P. corroboree are also characterized by indeterminate growth (as are most reptiles and amphibians) and thus likely have ample time and opportunity to strategically allocate resources to sperm production at the expense of growth and/or other sexually selected traits. Such strategic allocation could account for the intriguing lack of correlation between male age and body size in this study population (older males were not necessarily larger). An alternative explanation is that larger males may be too heavy to support during amplexus, making it difficult for females to oviposit optimally, resulting in lower fertilization success. This has been reported in the smooth toadlet (U. laevigata) where heavy males (> 70% of female body weight) impede successful fertilization, due to the inability of females to carry them during prolonged and arduous oviposition (Robertson 1990). In P. corroboree, oviposition is presumed to occur over a protracted period of at least several hours (possibly longer) and may even include a period of pre-ovipositional clasping (which may also last several hours), as reported in three other closely related, terrestrial breeding species, Pseudophryne bibronii, Pseudophryne dendyi, and Pseudophryne semimarmorata (Woodruff 1976). In this study, the average P. corroboree male was approximately 66% of the average female’s body weight. Thus, it seems plausible that P. corroboree males above a certain size threshold may be too large for the majority of females to support during this period of lengthy amplexus, reducing fertilization success. Overall, regardless of the mechanism, our results show that multiple traits may reflect a male’s fertilization capacity and suggest that female mate choice decisions may be based on male fertility.
While our findings shed light on patterns of mate choice in P. corroboree, there are some limitations that should be mentioned. First, it is possible that routine checks of male nests may have resulted in an increase in male movement and that some level of nest swapping may have occurred in between checks, adding noise to the data. Second, it should be recognized that we did not examine maternal effects. Although our design minimized the potential for such effects by randomizing the allocation of females to breeding tanks (and ensured that each tank received females across a range of body sizes), we were unable to explicitly test whether different female phenotypes had an influence on fertilization success or embryo development. There is also the potential for fertilization success to have been influenced by male–female interactive effects, with certain phenotypic combinations having more pronounced impacts on reproductive outcomes (Byrne et al. 2021). Additionally, we were unable to assess whether there were any male-male effects or female-female effects on mating success. In regard to male-male effects, intra-sexual competition could impact female access to preferred males and the allocation of eggs to certain males, but not others. Male P. corroboree rarely engage in the types of physically aggressive behavior frequently observed in other anurans that are subject to intense male-male competition (e.g., wrestling, grappling, ramming) (Byrne and Roberts 2004; Wells 2007; de Sa et al. 2020). Although it is possible that male P. corroboree may interfere with each other’s calling behavior and courtship by altering their call type (e.g., by producing more territorial calls), or by adjusting specific call characteristics (e.g., by strategically elevating their call rate in the presence of females) (Schwartz et al. 2001; Byrne 2008). Such dynamic vocal interactions between neighboring males could potentially influence the relative attractiveness of particular males within tanks and modify female mate choices (Schwartz et al. 2001). In regard to female-female interactions, aggression has never been observed in female P. corroboree, though interference could take more subtle forms, such as females monopolizing a male by remaining in amplexus for extended periods. However, the fact that there is a mating bias towards a subset of males indicates that there is unlikely to be any major constraint on females mating with preferred males. This bias, however, does raise the possibility that females copy mate choices of other females, as reported for various taxa (reviewed in Witte et al. 2015). Moving forward, controlling for maternal effects, and potential effects of courtship interference, should be factored into future studies investigating mate choice in P. corroboree.
Broad implications
Taken together, our results reveal that under captive conditions, female P. corroboree use multiple male traits within a sensory modality (multicomponent signal) to select mates and that fertilization success is predicted by multiple traits (both acoustic and physical traits). We expect that this is also likely to be the case in nature, as our recent field study in the closely related P. pengilleyi revealed that male mating success was predicted by multiple male traits (pulse repetition rate and age) (Kelleher et al. 2021a). Moreover, our results show that multiple male traits predict fertilization success, suggesting that P. corroboree females may gain direct fertility benefits from their mate choice decisions. This knowledge significantly advances our understanding of sexual selection processes in P. corroboree. Our findings also advance our understanding of the direct benefits gained from mate choice in anurans, as it is one of the first studies to demonstrate a link between male call traits (such as frequency and call rate) and fertilization success in a frog. More broadly, our findings contribute to a rapidly growing body of evidence in mammals, birds, anurans, fish, and invertebrates, showing that female mate choice is often based on suites of traits (Candolin 2003; Stange et al. 2017; Berson and Simmons 2018; Gould and Augustine 2020). Our study also draws attention to the complexity of female mate choice in highly dynamic acoustic environments (such as anuran breeding choruses) (Richardson and Lengagne 2010), highlighting that a single trait approach to understanding mate choice is unlikely to accurately reflect sexual selection processes (Starnberger et al. 2014).
Conservation implications
Our findings show that multiple male phenotypic traits predict successful breeding in a captive environment. We propose that this information may be used to develop behavior-based captive breeding protocols in P. corroboree. Currently, the P. corroboree captive breeding protocol involves offering females a choice between 2 and 8 males based on a maximum avoidance of inbreeding (MAI) genetic breeding scheme (OEH 2012). Based on our results, we suggest that conservation managers consider manipulating the composition of males available to females in order to bolster breeding success. For instance, it may be beneficial to increase the relative number of older males, or smaller males presented to females, to increase the fertilization success of clutches. Another possibility is that conservation managers may be able to manipulate male attractiveness by using knowledge of acoustic signals. By understanding which call traits are attractive, conservation managers may be able to predict which individual males are likely to be preferred by females. Males could then be strategically arranged in breeding choruses to either increase or decrease the relative attractiveness of individual males, and subsequently their probability of obtaining mating success. It may also be possible to construct “artificial” choruses using playback speakers where call traits of “neighboring” males are manipulated to increase the relative attractiveness of unmated males (e.g., using an approach similar to Lea and Ryan 2015). It is important to note that although P. corroboree can be bred successfully in captivity, integrating knowledge of mate choice has the potential to enhance total reproductive output by encouraging a greater proportion of females to mate, partitioning mating success among a larger pool of males, and increasing the proportion of viable, fertilized eggs. Perhaps more importantly, these types of behavioral manipulations may assist with the genetic management of P. corroboree, by facilitating the breeding of “unattractive” but genetically valuable males. This approach may increase the total number and genetic diversity of offspring available for reintroduction into the wild. Though it should be kept in mind that selective pressures in captivity may differ from those experienced in the wild, necessitating some consideration of genetic and phenotypic predictors of post-release performance and survival. Manipulation of signals used in mate choice to improve captive breeding output has only been achieved in a few mammal species (Roberts and Gosling 2004; Parrott et al. 2019), so its application to amphibian captive breeding programs remains novel and promising.
Our findings also have implications for the application of female mate choice to conservation breeding programs globally. Our results highlight the need for empiricists to consider how multiple signal components, across various sensory modalities, may interact to influence mate choice and subsequent reproductive outcomes within CBPs. For example, if conservation managers attempt to pair individuals for breeding based on knowledge of one signal component that influences mate choice, yet females actually require multiple signals to accurately assess potential mates, breeding pairs may still be incompatible, leading to reduced reproductive success (Noer et al. 2017). Moreover, empiricists may also need to take into account how specific captive breeding environments may influence which traits are important. For instance, in homogeneous captive breeding environments, females may place greater emphasis on male phenotypic traits as opposed to variation in resource quality (such as nest sites) (Lifjeld and Slagsvold 1988). Overall, we recommend that future research aiming to incorporate mate choice into CBPs takes a multivariate approach and identifies how multiple traits interact to influence male attractiveness before integrating knowledge of female mate choice into captive management.
Data availability
Data is available as supplementary material.
Code availability
Not applicable.
References
Andersson MB (1994) Sexual selection. Princeton University Press, Princeton, NJ, USA
Asa CS, Traylor-Holzer K, Lacy RC (2011) Can conservation-breeding programmes be improved by incorporating mate choice? Int Zoo Yearb 45:203–212
Bartoń K (2019) MuMIn: multi-model inference. R package version 1.42.15, https://CRAN.R-project.org/package=MuMIn
Berson JD, Simmons LW (2018) Sexual selection across sensory modalities: female choice of male behavioral and gustatory displays. Behav Ecol 29:1096–1104
Berven KA (1987) The heritable basis of variation in larval developmental patterns within populations of the wood frog (Rana sylvatica). Evolution 41:1088–1097
Brenes-Soto A, Dierenfeld ES, Janssens GPJ (2017) Colouration in amphibians as a reflection of nutritional status: the case of tree frogs in Costa Rica. PLoS ONE 12:e0182020
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM (2017). glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. The R Journal 9(2):378–400. https://journal.r-project.org/archive/2017/RJ-2017-066/index.html
Brooks R, Kemp DJ (2001) Can older males deliver the good genes? Trends Ecol Evol 16:308–313
Burke EJ, Murphy CG (2007) How female barking treefrogs, Hyla gratiosa, use multiple call characteristics to select a mate. Anim Behav 74:1463–1472
Bussière LF, Clark AP, Gwynne DT (2005) Precopulatory choice for cues of material benefits in tree crickets. Behav Ecol 16:255–259
Byrne PG (2008) Strategic male calling behavior in an Australian terrestrial toadlet (Pseudophryne bibronii). Copeia 2008:57–63
Byrne PG, Keogh JS (2009) Extreme sequential polyandry insures against nest failure in a frog. Proc R Soc Lond B 276:115–120
Byrne PG, Keogh JS, O’Brien DM, Gaitan-Espitia JD, Silla AJ (2021) Evidence that genetic compatability underpins female mate choice in a monandrous amphibian. Evolution 75:529–541
Byrne PG, Roberts JD (2004) Intrasexual selection and group spawning in quacking frogs (Crinia georgiana). Behav Ecol 15:872–882
Byrne PG, Roberts JD, Simmons LW (2002) Sperm competition selects for increased testes mass in Australian frogs. J Evol Biol 15:347–355
Cadena V, Rankin K, Smith KR, Endler JA, Stuart-Fox D (2018) Temperature-induced colour change varies seasonally in bearded dragon lizards. Biol J Linn Soc 123:422–430
Cadena V, Smith KR, Endler JA, Stuart-Fox D (2017) Geographic divergence and colour change in response to visual backgrounds and illumination intensity in bearded dragons. J Exp Biol 220:1048–1055
Cally JG, Stuart-Fox D, Holman L (2019) Meta-analytic evidence that sexual selection improves population fitness. Nat Commun 10:2017
Candolin U (2003) The use of multiple cues in mate choice. Biol Rev 78:575–595
Castellano S, Rosso A (2007) Female preferences for multiple attributes in the acoustic signals of the Italian treefrog, Hyla intermedia. Behav Ecol Sociobiol 61:1293–1302
Chargé R, Teplitsky C, Sorci G, Low M (2014) Can sexual selection theory inform genetic management of captive populations? A review. Evol Appl 7:1120–1133
Daly JW, Spande TF, Garraffo HM (2005) Alkaloids from amphibian skin: a tabulation of over eight-hundred compounds. J Nat Prod 68:1556–1575
de Sa FP, Consolmagno R, Muralidhar P, Brasileiro CA, Zamudio KR, Haddad C (2020) Unexpected reproductive fidelity in a polygynous frog. Sci Adv 6:eaay1539
Dreher CE, Pröhl H (2014) Multiple sexual signals: calls over colors for mate attraction in an aposematic, color-diverse poison frog. Front Ecol Evol 2:22
Dreher CE, Rodríguez A, Cummings ME, Pröhl H (2017) Mating status correlates with dorsal brightness in some but not all poison frog populations. Ecol Evol 7:10503–10512
Dziminski MA, Roberts JD, Simmons LW (2010) Sperm morphology, motility and fertilisation capacity in the myobatrachid frog Crinia georgiana. Reprod Fertil Dev 22:516–522
Edwards CL, Byrne PG, Harlow P, Silla AJ (2017) Dietary carotenoid supplementation enhances the cutaneous bacterial communities of the critically endangered southern corroboree frog (Pseudophryne corroboree). Microbial Ecol 73:435–444
Endler JA (1990) On the measurement and classification of colour in studies of animal colour patterns. Biol J Linn Soc 41:315–352
Evans JP (2010) Quantitative genetic evidence that males trade attractiveness for ejaculate quality in guppies. Proc R Soc Lond B 277:3195–3201
Evans JP, García-González F, Marshall DJ (2007) Sources of genetic and phenotypic variance in fertilization rates and larval traits in a sea urchin. Evolution 61:2832–2838
Felton A, Alford RA, Felton AM, Schwarzkopf L (2006) Multiple mate choice criteria and the importance of age for male mating success in the microhylid frog, Cophixalus ornatus. Behav Ecol Sociobiol 59:786–795
Flannery EW, Butts IAE, Stowinska M, Ciereszko A, Pitcher TE (2013) Reproductive investment patterns, sperm characteristics, and seminal plasma physiology in alternative reproductive tactics of Chinook salmon (Oncorhynchus tshawytscha). Biol J Linn Soc 108:99–108
Forsman A, Hagman M (2006) Calling is an honest indicator of paternal genetic quality in poison frogs. Evolution 60:2148–2157
Gerhardt HC (2001) Acoustic communication in two groups of closely related treefrogs. Adv Stud Behav 30:99–167
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans. The University of Chicago Press, Chicago
Gomez D, Richardson C, Lengagne T, Plenet S, Joly P, Léna JP, Théry M (2009) The role of nocturnal vision in mate choice: females prefer conspicuous males in the European tree frog (Hyla arborea). Proc R Soc Lond B 276:2351–2358
Gould GM, Augustine JK (2020) Multiple signals predict male mating success in the lek-mating lesser prairie-chicken (Tympanuchus pallidicinctus). Behav Ecol Sociobiol 74:137
Hartnett CM, Parrott ML, Mulder RA, Coulson G, Magrath MJL (2018) Opportunity for female mate choice improves reproductive outcomes in the conservation breeding program of the eastern barred bandicoot (Perameles gunnii). Appl Anim Behav Sci 199:67–74
Hunter D (2000) The conservation and demography of the southern corroboree frog (Pseudophryne corroboree). MSc thesis, University of Canberra
Hunter DA, Speare R, Marantelli G, Mendez D, Pietsch R, Osborne W (2010) Presence of the amphibian chytrid fungus Batrachochytrium dendrobatidis in threatened corroboree frog populations in the Australian alps. Dis Aquat Org 92:209–216
Jennions MD, Backwell PRY, Passmore NI (1995) Repeatability of mate choice: the effect of sizeoin the African painted reed frog, Hyperolius marmoratus. Anim Behav 49:181–186
Jennions MD, Petrie M (1997) Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev 72:283–327
Kelleher SR, Scheele BC, Silla AJ, Keogh JS, Hunter DA, Byrne PG (2021a) Disease influences male advertisement and mating outcomes in a critically endangered amphibian. Anim Behav 173:145–157
Kelleher SR, Silla AJ, Hertel AG, Dingemanse NJ, Byrne PG (2021b) Mate preference plasticity in a critically endangered frog: implications for conservation breeding. Front Conserv Sci. 2:748104
Klaus SP, Fitzsimmons LP, Pitcher TE, Bertram SM (2011) Song and sperm in crickets: a trade-off between pre- and post-copulatory traits or phenotype-linked fertility? Ethology 117:154–162
Klump GM, Gerhardt HC (1987) Use of non-arbitrary acoustic criteria in mate choice by female gray tree frogs. Nature 326:286–288
Kokko H, Brooks R, Jennions MD, Morley J (2003) The evolution of mate choice and mating biases. Proc R Soc Lond B 270:653–664
Lea AM, Ryan MJ (2015) Irrationality in mate choice revealed by túngara frogs. Science 349:964–966
Lesbarrères D, Lodé T (2002) Variations in male calls and responses to an unfamiliar advertisement call in a territorial breeding anuran, Rana dalmatina: Evidence for a “dear enemy” effect. Ethol Ecol Evol 14:287–295
Lesbarrères D, Merilä J, Lodé T (2008) Male breeding success is predicted by call frequency in a territorial species, the agile frog (Rana dalmatina). Can J Zool 86:1273–1279
Lifjeld JT, Kleven O, Jacobsen F, Mc Graw KJ, Safran RJ, Robertson RJ (2011) Age before beauty? Relationships between fertilization success and age-dependent ornaments in barn swallows. Behav Ecol Sociobiol 65:1687–1697
Lifjeld JT, Slagsvold T (1988) Female pied flycatchers Ficedula hypoleuca choose male characteristics in homogeneous habitats. Behav Ecol Sociobiol 22:27–36
Locatello L, Rasotto MB, Evans JP, Pilastro A (2006) Colourful male guppies produce faster and more viable sperm. J Evol Biol 19:1595–1602
Lüpold S, Simmons LW, Grueter CC (2019) Sexual ornaments but not weapons trade off against testes size in primates. Proc R Soc B 286:2182542
Maan ME, Cummings ME (2009) Sexual dimorphism and directional sexual selection on aposematic signals in a poison frog. P Natl Acad Sci USA 106:19072–19077
Martin-Wintle MS, Shepherdson D, Zhang G, Zhang H, Li D, Zhou X, Li R, Swaisgood RR (2015) Free mate choice enhances conservation breeding in the endangered giant panda. Nat Commun 6:10125
Martin-Wintle MS, Wintle NJP, Díez-León M, Swaisgood RR, Asa CS (2019) Improving the sustainability of ex situ populations with mate choice. Zoo Biol 38:119–132
McFadden M, Hobbs R, Marantelli G, Harlow P, Banks C, Hunter D (2013) Captive management and breeding of the critically endangered southern corroboree frog (Pseudophryne corroboree) (Moore 1953) at Taronga and Melbourne Zoos. Amphib Reptile Conse 5:70–87
McInerney EP, Silla AJ, Byrne PG (2019) Effect of carotenoid class and dose on the larval growth and development of the critically endangered southern corroboree frog. Conserv Physiol 7:coz009
Mitoyen C, Quigley C, Fusani L (2019) Evolution and function of multimodal courtship displays. Ethology 125:503–515
Montoya B, Torres R (2015) Male skin color signals direct and indirect benefits in a species with biparental care. Behav Ecol 26:425–434
Morris MR, Yoon SL (1989) A mechanism for female choice of large males in the treefrog Hyla chrysoscelis. Behav Ecol Sociobiol 25:65–71
Neff BD, Pitcher TE (2005) Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol Ecol 14:19–38
Noer CL, Balsby TJS, Anistoroaei R, Stelvig M, Dabelsteen T (2017) Mate choice screening in captive solitary carnivores: the role of male behavior and cues on mate preference and paternity in females of a model species, American mink (Neovison vison). Zoo Biol 36:367–381
O’Brien DM, Silla AJ, Forsythe PS, Byrne PG (2021) Sex differences in response to environmental and social breeding cues in an amphibian. Behaviour 158:397–426
OEH (2012) National Recovery Plan for the Southern Corroboree frog, Pseudophryne corroboree, and the Northern Corroboree Frog Pseudophryne pengilleyi. Office of Environment and Heritage (NSW), Hurstville, NSW
Osborne WS (1991) The biology and management of the Corroboree Frog (Pseudophryne corroboree) in NSW. NSW National Parks and Wildlife Service, Hurstville, NSW
Parrott ML, Nation A, Selwood L (2019) Female mate choice significantly increases captive breeding success, and scents can be frozen to determine choice, in the stripe-faced dunnart. Appl Anim Behav Sci 214:95–101
Pengilley RK (1971) Calling and associated behaviour of some species of Pseudophryne (Anura: Leptodactylidae). J Zool 163:73–92
Pengilley RK (1973) Breeding biology of some species of Pseudophryne (Anura: Leptodactylidae) of the Southern Highlands, New South Wales. Aust Zool 18:15–30
Petrie M (1994) Improved growth and survival of offspring of peacocks with more elaborate trains. Nature 371:598–599
Pröhl H (2003) Variation in male calling behaviour and relation to male mating success in the strawberry poison frog (Dendrobates pumilio). Ethology 109:273–290
Pujolar JM, Locatello L, Zane L, Mazzoldi C (2012) Body size correlates with fertilization success but not gonad size in grass goby territorial males. PLoS ONE 7:e46711
Quader S (2005) Mate choice and its implications for conservation and management. Curr Sci 89:1220–1229
R Development Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
Rausch AM, Sztatecsny M, Jehle R, Ringler E, Hödl W (2014) Male body size and parental relatedness but not nuptial colouration influence paternity success during scramble competition in Rana arvalis. Behaviour 151:1869–1884
Reaney LT, Backwell PRY (2007) Temporal constraints and female preference for burrow width in the fiddler crab, Uca mjoebergi. Behav Ecol Sociobiol 61:1515–1521
Richardson C, Lengagne T (2010) Multiple signals and male spacing affect female preference at cocktail parties in treefrogs. Proc R Soc Lond B 277:1247–1252
Roberts SC, Gosling LM (2004) Manipulation of olfactory signaling and mate choice for conservation breeding: a case study of harvest mice. Conserv Biol 18:548–556
Robertson JGM (1990) Female choice increases fertilization success in the Australian frog, Uperoleia laevigata. Anim Behav 39:639–645
Rosenthal GG (2017) Mate choice: the evolution of sexual decision making from microbes to humans. Princeton University Press, Princeton
Rosenthal MF, Hebets EA (2015) Temporal patterns of nutrition dependence in secondary sexual traits and their varying impacts on male mating success. Anim Behav 103:75–82
Ryan MJ, Keddy-Hector A (1992) Directional patterns of female mate choice and the role of sensory biases. Am Nat 139:S4–S35
Ryan MJ, Page RA, Hunter KL, Taylor RC (2019) ‘Crazy love’: nonlinearity and irrationality in mate choice. Anim Behav 147:189–198
Scarponi V, Godin J-GJ (2018) Female assessment of male functional fertility during mate choice in a promiscuous fish. Ethology 124:196–208
Schmoll T, Mund V, Dietrich-Bischoff V, Winkel W, Lubjuhn T (2007) Male age predicts extrapair and total fertilization success in the socially monogamous coal tit. Behav Ecol 18:1073–1081
Schwartz JJ, Buchanan BW, Gerhardt HC (2001) Female mate choice in the gray treefrog (Hyla versicolor) in three experimental environments. Behav Ecol Sociobiol 49:443–455
Settle RA, Ettling JA, Wanner MD, Schuette CD, Briggler JT, Mathis A (2018) Quantitative behavioral analysis of first successful captive breeding of endangered Ozark hellbenders. Front Ecol Evol 6:205
Sheldon BC (1994) Male phenotype, fertility, and the pursuit of extra-pair copulations by female birds. Proc R Soc Lond B 257:25–30
Sheldon BC, Arponen H, Laurila A, Crochet PA, Merilä J (2003) Sire coloration influences offspring survival under predation risk in the moorfrog. J Evol Biol 16:1288–1295
Sherman CDH, Sagvik J, Olsson M (2010) Female choice for males with greater fertilization success in the Swedish moor frog. Rana arvalis. PLoS ONE 5:e13634
Silla AJ, McInerney EP, Byrne PG (2016) Dietary carotenoid supplementation improves the escape performance of the southern corroboree frog. Anim Behav 112:213–220
Simmons LW, Lüpold S, Fitzpatrick JL (2017) Evolutionary trade-off between secondary sexual traits and ejaculates. Trends Ecol Evol 32:964–976
Simmons LW, Tinghitella RM, Zuk M (2010) Quantitative genetic variation in courtship song and its covariation with immune function and sperm quality in the field cricket Teleogryllus oceanicus. Behav Ecol 21:1330–1336
Smith MJ, Roberts JD (2003a) An experimental examination of female preference patterns for components of the male advertisement call in the quacking frog, Crinia georgiana. Behav Ecol Sociobiol 55:144–150
Smith MJ, Roberts JD (2003b) Call structure may affect male mating success in the quacking frog, Crinia georgiana (Anura: Myobatrachidae). Behav Ecol Sociobiol 53:221–226
Stange N, Page RA, Ryan MJ, Taylor RC (2017) Interactions between complex multisensory signal components result in unexpected mate choice responses. Anim Behav 134:239–247
Starnberger I, Preininger D, Hödl W (2014) From uni- to multimodality: towards an integrative view on anuran communication. J Comp Physiol A 200:777–787
Stearns S (1989) Trade-offs in life-history evolution. Funct Ecol 3:259–268
Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21
Taylor RC, Buchanan BW, Doherty JL (2007) Sexual selection in the squirrel treefrog Hyla squirella: the role of multimodal cue assessment in female choice. Anim Behav 74:1753–1763
Umbers KDL, Silla AJ, Bailey JA, Shaw AK, Byrne PG (2016) Dietary carotenoids change the colour of Southern corroboree frogs. Biol J Linn Soc 19:436–444
Wagner WE Jr, Harper CJ (2003) Female life span and fertility are increased by the ejaculates of preferred males. Evolution 57:2054–2066
Walls SC, Gabor CR (2019) Integrating behavior and physiology into strategies for amphibian conservation. Front Ecol Evol 7:234
Walton SJ, Silla AJ, Endler JA, Byrne PG (2021) Does dietary β-carotene influence ontogenetic colour change in the southern corroboree frog? J Exp Biol 224:jeb243182
Welch AM, Semlitsch RD, Gerhardt HC (1998) Call duration as an indicator of genetic quality in male gray tree frogs. Science 280:1928–1930
Wells K (2007) The ecology and behaviour of amphibians. The University of Chicago Press, London, UK
Wielebnowski NC (1998) Contributions of behavioral studies to captive management and breeding of rare and endangered mammals. In: Caro T (ed) Behavioral Ecology and Conservation Biology. Oxford University Press Inc., New York, pp 130–162
Witte K, Kniel N, Kureck M (2015) Mate-choice copying: status quo and where to go. Curr Zool 61:1073–1081
Woodruff DS (1976) Courtship, reproductive rates, and mating system in three Australian Pseudophryne (Amphibia, Anura, Leptodactylidae). J Herpetol 10:313–318
Young B, Conti DV, Dean MD (2013) Sneaker ‘jack’ males outcompete dominant ‘hooknose’ males under sperm competition in Chinook salmon (Oncorhynchus tshawytscha). Ecol Evol 3:4987–4997
Zippel K, Johnson K, Gagliardo R, Gibson R, McFadden M, Browne R, Martinez C, Townsend E (2011) The amphibian Ark: a global community for ex situ conservation of amphibians. Herpetol Conserv Biol 6:340–352
Zuur A, Ieno E, Walker N, Saveliev A, Smith G (2009) Mixed effects models and extensions in ecology with R. Springer Science and Business Media, New York, USA
Acknowledgements
We thank Emma McInerney for assistance with data collection. We also thank Associate Professor Alexander Taylor Baugh, and four anonymous reviewers for their constructive comments which improved the quality of the article. The authors thank staff from Taronga Conservation Society Australia’s Herpetofauna Division, in particular Emma Bembrick, Caitlin Gilchrist, and Cara Anger for assistance with amphibian husbandry. The authors also acknowledge the support of the NSW Department of Planning, Industry, and Environment Threatened Species Officer, Dr David Hunter, and members of the Corroboree Frog Recovery Team.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The study was funded by the Australian Research Council (Linkage Grant LP170100351) awarded to PGB and AJS, and the University of Wollongong SMAH Small Project Grant (262 27 0976) awarded to PGB and AJS. This study was also supported by the Holsworth Research Endowment-Equity Trustees Charitable Foundation and the Ecological Society of Australia, and a Frog and Tadpole Study Group of New South Wales student grant awarded to SRK. This work was conducted while SRK was in receipt of an Australian Government Research Training Program (RTP) Scholarship.
Author information
Authors and Affiliations
Contributions
PGB, AJS, and SRK conceived the study. SRK and MSM collected the data. JAE developed color quantification methods and designed and wrote the color analysis program. SRK analyzed the call and color data. SRK and MGS ran the statistical analyses. SRK wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Ethics approval
All work conducted during this study was approved by the Taronga Conservation Society Australia Animal Ethics Committee (AEC protocol number—3a/10/16). The nest checks conducted during this study are in line with the current corroboree frog breeding protocol, and all viable eggs obtained from this study were reintroduced into the wild as part of the corroboree frog recovery plan.
Consent for publication
All authors have given their consent for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by A. Taylor Baugh.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kelleher, S.R., Silla, A.J., McFadden, M.S. et al. Multiple phenotypic traits predict male mating success in a critically endangered frog. Behav Ecol Sociobiol 76, 40 (2022). https://doi.org/10.1007/s00265-021-03119-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-021-03119-9