Abstract
Artificial light at night is increasing globally, interfering with both sensory ecology and temporal rhythms of organisms, from zooplankton to mammals. This interference can change the behaviour of the affected organisms, and hence compromise the viability of their populations. Limiting the use of artificial light may mitigate these negative effects. Accordingly, we investigated whether the duration of artificial light affects sexual signalling in female glow-worms, Lampyris noctiluca, which are flightless and attract flying males to mate by emitting glow that is interfered by light pollution. The study included three treatments: no artificial light (control), 15 min of artificial light, and 45 min of artificial light. The results show that females were more likely to cease glowing when the exposure to light was longer. Furthermore, small females were more likely to cease their glow, and responded faster to the light, than larger females. These findings suggest that glow-worms can react rapidly to anthropogenic changes in nocturnal light levels, and that prolonged periods of artificial light trigger females to stop sexual signalling. Thus, limiting the duration of artificial light can mitigate the adverse effects of light pollution on sexual signalling, highlighting the importance of such mitigation measures.
Significance statement
Interest in the effects of artificial light at night on animal behaviour has increased in recent years. With evidence for its negative impact accumulating, potential remedies, such as limiting the duration of light exposure, have emerged. To date, however, knowledge on the effectiveness of these methods has remained very limited. We show that female European common glow-worms, which are wingless beetles that glow to attract flying males to mate, responded to prolonged artificial light exposure by discontinuing their glow. Such non-glowing females are not expected to find a mate, making it difficult for them to reproduce. Hence, our study indicates that the duration of artificial light should be limited to protect this night-active beetle and its opportunities for effective sexual signalling. Because many other nocturnal species also need darkness, this study provides valuable information for the development and use of less disruptive night-time lights.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural environments are increasingly affected by human activity. Indeed, anthropogenic disturbances, such as noise, light pollution, and habitat destruction, have become more common and increased in their intensity (Gaston et al. 2015, 2021; Swaddle et al. 2015). Many organisms struggle with adapting to such quick environmental changes (Wong and Candolin 2015). Indeed, when an organism is exposed to novel conditions that are different from those of its recent evolutionary past, it may lack the phenotypic or genetic variation to react appropriately. This is also true regarding signals, such as sounds and physical ornaments, that animals use to attract mates. If a novel anthropogenic disturbance closely resembles the signal, or otherwise affects pre-existing signal–response patterns, its impacts on the signallers and receivers are likely to be particularly severe (Sih et al. 2011; Wong and Candolin 2015). Reproductive success of signallers and signal receivers in such altered environments depends on how well they succeed in adjusting, and possibly genetically adapting, to the new conditions. Common strategies, in this regard, include either avoiding or overriding the disturbance. For example, urban moths are less inclined to fly towards artificial light sources compared to their rural counterparts (Altermatt and Ebert 2016), while certain birds rapidly alter the pitch of their vocal signalling in response to noise (Slabbekoorn 2013), and Aquatica ficta fireflies emit brighter flash signals in response to artificial light (Owens et al. 2018). The likelihood of such adjustments taking place is likely to depend on the phenotypic plasticity and sensory ecology of the organism (Sih et al. 2011; Tuomainen and Candolin 2011).
Artificial lights, such as street, commercial, safety, and vehicle lights, alter the nocturnal environment by changing the spectral, spatial, and temporal distribution of ambient light (Gaston et al. 2015). In addition, artificial light sources usually display considerably higher light intensities (10–60 lx) compared to natural nightly light sources, such as the moon (typically 0.1–0.6 lx; Kyba et al. 2017). Light pollution is known to affect a range of taxa, including mammals, birds, insects, and zooplankton, by disrupting their temporal rhythms or directly interfering with their senses (Sih et al. 2011; Gaston et al. 2015; Dominoni and Nelson 2018). Despite the increasing interest in the effects of light pollution, many aspects of its consequences are still poorly understood (Spoelstra et al. 2015; Boyes et al. 2020), highlighting the need for both additional research and mitigation measures. As light pollution affects wildlife in many ways, various solutions to the growing problem have been suggested. Most commonly, the recommendations have included reduction of the lit area, adjustment of the spectral properties of light sources, the use of less light for shorter periods of time (motion sensors or part-night lighting), and the use of light fixtures that minimise the spread of scattered light into the environment (Gaston et al. 2012; Longcore and Rich 2016). These approaches seem intuitive, but experimental research on their effectiveness for limiting the impact of artificial light at night is lagging behind (for work on bats, see Azam et al. 2015; Day et al. 2015).
Glow-worms and flashing fireflies use bioluminescent signals to attract mates in the dark and are thus likely to be especially vulnerable to light pollution (Lewis 2016). In these insects, the initiation of signalling is highly susceptible to changes in ambient light (Schwalb 1961; Dreisig 1971, 1975, 1978), and hence artificial light can hamper their mate attraction and even reproductive success (Ineichen and Rüttimann 2012; Owens and Lewis 2018; Elgert et al. 2020, 2021; Van den Broeck et al. 2021b). Thus, glow-worms and other fireflies may be threatened by the global increase in the use of artificial light, as well as by other factors, such as habitat loss, climate change, and chemical pollution (Lewis et al. 2020; Owens et al. 2020). Some species, such as the common glow-worm, Lampyris noctiluca, have been declining at least locally (Gardiner and Didham 2020), and while they may be negatively affected by light pollution (Elgert et al. 2020; Lewis et al. 2020; Van den Broeck et al. 2021b), the effects of the duration of artificial light on their behaviour remain poorly understood. Therefore, it is important to investigate how these charismatic beetles handle light pollution and whether the negative effects could be mitigated.
Accordingly, we investigated how the length of exposure to artificial light affects the glow that flightless glow-worm females use to attract males to mate. Earlier studies revealed that streetlights hamper the capacity of female glow-worms (both actual females and green LED dummies) to attract males, and that females usually do not move away from constant light, but instead glow less (Bird and Parker 2014; Elgert et al. 2020; Van den Broeck et al. 2021a). To test whether and how females react to different periods of time spent under artificial light, we designed a laboratory experiment to test their responses. We expected responses of female glow-worms to become stronger with the length of exposure to artificial light.
Materials and methods
In the common glow-worm, sedentary and wingless females try to attract flying males, with brighter and larger females typically being more successful (Tyler 2002; Hopkins et al. 2015). Female body size, in turn, is defined during the larval stage by factors such as food availability, the physical environment, genetic factors, and larval development time (Tyler 2002). Adult females start to glow at sunset, when the ambient light level has decreased to a low enough level, and continue to glow for a couple of hours per night until they either succeed in mating or die (Schwalb 1961; Dreisig 1971; Tyler 2002). The distinct green/yellow glow (546–570 nm) is produced by a chemical reaction in the lantern on the underside of the last abdominal segments (Tyler 2002). As capital breeders, adult glow-worms have a limited amount of resources to allocate to survival and reproduction, with the fecundity of unmated females quickly decreasing over time (Tyler 2002; Hopkins et al. 2021). In Finland, at the northern limit of their range (Borshagovski et al. 2020), glow-worm peak reproductive season is in June–July (Borshagovski et al. 2020; personal observations), when nights are short. Interestingly, previous research suggests that both female size and the intensity of female glow increase with latitude, presumably as local adaptations to the higher levels of ambient light during summer nights (Borshagovski et al. 2020).
We collected glow-worm females for the experiment in June–July 2019 from the surroundings of Tvärminne Zoological Station, Southern Finland (N 59° 51′, E 23° 14′). The females were transported to the laboratory and placed in individual vials (diameter: 8 cm, height: 4 cm), each with a mesh cover for air exchange, and fresh moss and leaves to prove moisture and shelter. The vials with females were kept in a non-insulated shed with a see-through roof, thus experiencing natural temperature and light conditions (light/dark: ~ 20L/4D during the study). Females were used in the experiment on average 2.84 days (SE = 0.09, N = 94) after capture.
We investigated in a laboratory experiment how female glow-worms are affected by the period of time they are exposed to artificial light, when the light is turned on after they have started to glow. For this purpose, we tested 94 females, of which 88 started to glow during the experiment. The females that did not glow at any point during the experiment (N = 6), were excluded from all analyses, because the absence of glowing is likely to indicate either poor condition (being out of energy) or mating briefly before being collected (females cease to glow soon after mating, but not immediately). The experiment was started at 23.30 and completed at 01.30 (giving the total duration of 120 min). It had three treatments: control with no exposure to artificial light (N = 30 females); short exposure to artificial light, for 15 min (based on findings of a preliminary experiment) starting at 00.46 (N = 27); and long exposure to artificial light, for 45 min (hence running until the end of the experiment), starting at 00.46 (N = 31). Thus, in the light exposed groups, the light was turned on 75 min after the start of the experiment, giving the females a long undisturbed period to start their glow.
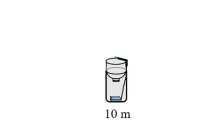
In the experimental arena, a source of light (cool white headlight, peak: 0.14 µW/cm2/nm at 455 nm), with an intensity of 15–20 lx that peaked in the centre of the beam, was placed straight above the focal female at the height of 180 cm (Fig. 1). This way, its intensity corresponded to that of a common streetlight (Gaston et al. 2012, 2015). We used two separate light sources in the experiment, which were assigned haphazardly across treatments and nights. We used tarpaulins to prevent light leakage between the treatments.
The setup of the experiment. Female glow-worms were placed in vials (one female per vial) and were allowed to start glowing before the artificial light was switched on. They were then exposed to one of three treatments: no light (control, N = 30), 15 min of light (short exposure, N = 27), and 45 min of light (long exposure, N = 31)
Each night, we haphazardly assigned 1 to 10 females to the three treatments, depending on how many females we had available. At the start of a trial, in all three treatments, the focal female was placed in a small vial (diameter: 8 cm, height: 12 cm), with a layer of soil on the bottom and a net covering the top to prevent the female from escaping. The walls of the vial were opaque, blocking all visual interactions between females tested at the same time. Each female was provided with one bivalve shell for shelter and two sticks for perching on. The female was placed in a vial in the experimental arena at approximately 23.00 to give it time to acclimatise to its surroundings until the start of the experiment at 23.30.
During the trial, we checked each female every 15 min and recorded when it started and ended to glow. If the onset of glowing was noticed at a point of time between the 15-min check points, this point of time was recorded as the starting time. We recorded these data conservatively: the female had to produce clearly visible glow to be recorded as having started to glow, and it had to stop completely to be recorded as having ceased its glow. When in any doubt, the observer briefly created a shadow to check whether the glow had completely ceased. To estimate body size, we recorded the pronotum width (first exoskeletal plate) of each female (Hopkins et al. 2015). We returned all females to the field after they had been tested.
All statistical analyses were conducted using R version 4.0.2 (https://www.R-project.org). The effects of treatment and female size on the time it took for females to discontinue their glow (measured in minutes since the start of the experiment) were analysed with an accelerated failure time model (AFT). The analysis was performed using the “survreg” function with a Weibull distribution in the “survival” package (version 3.1–12) (Therneau 2020). Fixed factors were body size (pronotum width), the light treatment, and the interaction between size and treatment. The number of days since capture was added as a covariate. If the interaction term was found to be non-significant (χ2–test, P > 0.1), we refitted the model without it.
To check that the behaviour of females did not differ between the three treatments already before the artificial light was turned on, we ran, for females that did start to glow (and hence were available for the above analysis), a GLM with a negative binomial error distribution (as appropriate for data that were overdispersed; glm.nb-function from the MASS package, version 7.3–51.6 (Venables and Ripley 2002)). We fitted the model with the period of time until the female started to glow (in full minutes since the beginning of the experiment) as the response variable, body size, treatment, and size × treatment interaction as fixed factors, and days since capture as a covariate. If the interaction term was found to be non-significant (χ2–test, P > 0.1), the model was refitted without it.
Results
The AFT model revealed that the body size × artificial light treatment interaction term was non-significant (Table 1), and the model was therefore refitted without it. In the final model, both artificial light and body size effects were significant, whereas the number of days spent in captivity was not (Table 1). The females exposed to 45 min of light (long exposure) were more prone to cease glowing than females in the control and those exposed to 15 min of artificial light (short exposure) (Table 1). The difference between the control and the short exposure treatments was not significant (Table 1). In particular, the probability of a female ceasing to glow was the lowest in the control (1/30 or 3%), followed by the short exposure time of 15 min (4/27 or 15%), and the long exposure time of 45 min (28/31 or 90%). The average time from the start of the trial at which the females ceased to glow was 101.4 min (SE = 2.0, N = 33), i.e. 26 min after the light was turned on. Small females were more likely to stop glowing, and did so more quickly, than large females (Fig. 2; Fig. 3; Table 1).
The latency until female glow-worms ceased to glow in the absence (control) and presence of artificial light for either a short (15 min) or long (45 min) period of time. The females exposed to 45 min of light (long exposure) were more prone to cease glowing than females in the control and those exposed to 15 min of artificial light (short exposure). The graph shows Kaplan–Meier survival curves for latency. ( +) indicates right-censored data
Interestingly, all 4 females that ceased to glow in the short exposure treatment restarted their glow after the light was turned off and before the trial had ended. None of the 28 females in the long exposure treatment restarted to glow before their trial was completed, probably because the light was not switched off before the end of the trial.
In all treatments, females typically started to glow before the time the artificial light was turned on in the exposure treatments (at 00.46), and, as expected, the treatment to which a female had been assigned did not affect the onset time of its glow (Table 2). However, pronotum width (proxy for body size) did influence the onset time (Table 2): larger females initiated their glow earlier than small ones (Table 2; Fig. 4). The covariate, time spent in captivity, did not significantly affect glow onset time (Table 2).
Discussion
Our study is among the first to demonstrate that the response of female glow-worms to artificial light depends on the length of time they are exposed to the light. The most notable reaction of females to artificial light was to cease glowing. Interestingly, a brief exposure to light was less detrimental than an extended one: the probability of glow discontinuation was significantly higher in the long exposure treatment than control or short exposure treatment, but it did not differ between the control and short exposure treatments.
We suggest that females responded to artificial light by ceasing to glow because in the wild female glow-worms use natural ambient light to time their glow (Schwalb 1961; Dreisig 1971; Tyler 2002). Such a glow strategy should work well under a natural light–dark cycle, when any bright light is associated with the sun. Artificial light, in contrast, is both a relatively novel environmental disturbance and typically much brighter than any natural nocturnal light. Therefore, unexpectedly bright light at night can, as shown by our results, trigger females to stop their glow, which they would under natural circumstances only do at the arrival of the dawn or following a successful mating. An alternative possibility is that females have evolved to respond by ceasing to signal when the light disturbance has continued long enough to be deemed relatively continuous and, hence, to constitute a major interference to the visibility of their glow signal. Because, under undisturbed natural conditions, there are no temporary light sources brighter than the moonlight, this second alternative would imply an evolved response to artificial light and therefore warrants future research.
After turning on the light, it took on average 26 min for a female to cease to glow. This delay before a reaction is in line with prior findings (Dreisig 1975). It can also explain earlier observations of females glowing under a streetlight (Ineichen and Rüttimann 2012), despite the probability of attracting a male under artificial light is significantly reduced (Ineichen and Rüttimann 2012; Bird and Parker 2014; Elgert et al. 2020, 2021; Van den Broeck et al. 2021b). In addition, females have not been found to move away from a source of light (Elgert et al. 2020), despite being in a hurry to mate (Hopkins et al. 2021). Instead, when exposed to artificial light before having started to glow, females glow less and hide more than under control conditions (Elgert et al. 2020). Interestingly, all females that stopped to glow in our short light exposure treatment restarted glowing after the disturbance was removed. This result is in accordance with observations by Schwalb (1961), according to which females resumed glowing soon after a strong light (500 lx) was turned off. These findings indicate that female glow-worms are probably able to recover from prematurely ending their glow, provided that the disturbance is brief.
Earlier studies show that female glow-worms are sensitive to light conditions and can resynchronize their circadian rhythm when light levels in the environment are artificially altered (Schwalb 1961; Dreisig 1978). Thus, temporary artificial light at night has the potential to shift the time window available for a successful mating later into the night. The magnitude of such a phase shift, in turn, can be expected to depend on the length of the light exposure. Because glow-worms are usually active only a few hours per night, and males typically start their mate searching after females have started to glow, and stop it earlier than females stop signalling (Schwalb 1961; Dreisig 1971; Tyler 2002), any shift in the timing of female glow presumably needs to be matched by a similar shift in male mate searching.
In this study, females varied regarding how long they continued to glow after the start of light exposure: some stopped almost immediately, while others continued to glow for well over 45 min under the light. One factor that explained these individual differences was female size (here measured as pronotum width, which varied between 2.47 and 4.34 mm), with small females reacting faster to the light than large ones. We also found that large females initiated their glow earlier. These results could be explained by larger females being brighter (Hopkins et al. 2015) and hence having a higher probability to attract males under artificial light than smaller females (Elgert et al. 2021). Conversely, small females may be able to attract mates only during the darkest hours of the night (Borshagovski et al. 2020). Given that small females start their glow later, and cease it more easily under light, artificial light shortens the time available for mate attraction relatively more in small than large females. Similarly, the effects of artificial light on the glow and its initiation may differ across latitudes (Dreisig 1978), with females presumably needing a larger size and brighter glow to attain an adequate visibility in higher latitudes (Borshagovski et al. 2020), providing an interesting topic for further research.
Glow-worms and other fireflies face many anthropogenic threats (Lewis et al. 2020), including climate change, pesticides, habitat fragmentation, road mortality, and pollution (including artificial light) (Lewis et al. 2020; Lehtonen et al. 2021). Our finding that extended exposure to artificial light causes female glow-worms to cease glowing suggests that glow-worm reproduction (especially mate searching) would benefit from minimisation of the period of time that an artificial light is turned on, easing the negative anthropogenic impact on these beetles. Glow-worms and flashing fireflies aside, at least 49.5% (with the exact figure depending on the method) of the world’s land surface between 59° N and 55° S, not restricted to urban areas, has been estimated to be exposed to either direct or indirect night-time light pollution (Gaston et al. 2021). Given that a range of species, from insects to mammals, show adverse responses to light pollution (Dominoni and Nelson 2018), limiting both the amount of nocturnal light sources and the duration of the light is likely to be beneficial. Indeed, earlier work on bats (Azam et al. 2015; Day et al. 2015) and pollinating moths (Macgregor et al. 2019), has given promising results regarding correctly timed part-night lighting, albeit its efficiency varies depending on the species.
To conclude, our study shows that light pollution disturbs sexual signalling in the common glow-worm, with the extent of this effect depending on both the duration of the exposure to the light and the body size of the affected female. Thus, we recommend limiting the duration of artificial light to under 25 min at a time, to light only crucial areas (preferably away from glow-worm habitats), and to use light sources that minimise scattered light. For example, motion sensor technology seems a promising method for decreasing the negative effects of light pollution. In this respect, our results provide valuable information for the development of lighting systems to mitigate the adverse effects of light pollution on organisms.
Data accessibility
The data is provided as supplementary material.
References
Altermatt F, Ebert D (2016) Reduced flight-to-light behaviour of moth populations exposed to long-term urban light pollution. Biol Lett 12:20160111. https://doi.org/10.1098/rsbl.2016.0111
Azam C, Kerbiriou C, Vernet A, Julien J-F, Bas Y, Plichard L, Maratrat J, Le Viol I (2015) Is part-night lighting an effective measure to limit the impacts of artificial lighting on bats? Global Change Biol 21:4333–4341. https://doi.org/10.1111/gcb.13036
Bird S, Parker J (2014) Low levels of light pollution may block the ability of male glow-worms (Lampyris noctiluca L.) to locate females. J Insect Conserv 18:737–743. https://doi.org/10.1007/s10841-014-9664-2
Borshagovski A-M, Saari P, Lehtonen TK, Kaitala A (2020) When night never falls: female sexual signalling in a nocturnal insect along a latitudinal gradient. Behav Ecol Sociobiol 74:153. https://doi.org/10.1007/s00265-020-02927-9
Boyes DH, Evans DM, Fox R, Parsons MS, Pocock MJO (2020) Is light pollution driving moth population declines? A review of causal mechanisms across the life cycle. Insect Conserv Divers:167–187. https://doi.org/10.1111/icad.12447
Day J, Baker J, Schofield H, Mathews F, Gaston KJ (2015) Part-night lighting: implications for bat conservation. Anim Conserv 18:512–516. https://doi.org/10.1111/acv.12200
Dominoni DM, Nelson RJ (2018) Artificial light at night as an environmental pollutant: an integrative approach across taxa, biological functions, and scientific disciplines. J Exp Zool A Ecol Integr Physiol 329:387–393. https://doi.org/10.1002/jez.2241
Dreisig H (1971) Control of glowing of Lampyris noctiluca in the field (Coleoptera: Lampyridae). J Zool 165:229–244. https://doi.org/10.1111/j.1469-7998.1971.tb02183.x
Dreisig H (1975) Environmental control of the daily onset of luminescent activity in glowworms and fireflies (Coleoptera: Lampyridae). Oecologia 18:85–99. https://doi.org/10.1007/BF00348090
Dreisig H (1978) The circadian rhythm of bioluminescence in the glowworm, Lampyris noctiluca L. (Coleoptera, Lampyridae). Behav Ecol Sociobiol 3:1–18. https://doi.org/10.1007/BF00300044
Elgert C, Hopkins J, Kaitala A, Candolin U (2020) Reproduction under light pollution: maladaptive response to spatial variation in artificial light in a glow-worm. Proc R Soc B 287:20200806. https://doi.org/10.1098/rspb.2020.0806
Elgert C, Lehtonen TK, Kaitala A, Candolin U (2021) Sexual selection for bright females prevails under light pollution. Curr Zool 67:329–331. https://doi.org/10.1093/cz/zoaa071
Gardiner T, Didham RK (2020) Glowing, glowing, gone? Monitoring long-term trends in glow-worm numbers in south-east England. Insect Conserv Divers 13:162–174. https://doi.org/10.1111/icad.12407
Gaston KJ, Ackermann S, Bennie J, Cox DTC, Phillips BB, Sánchez de Miguel A, Sanders D (2021) Pervasiveness of biological impacts of artificial light at night. Integr Comp Biol: in press. https://doi.org/10.1093/icb/icab145
Gaston KJ, Davies TW, Bennie J, Hopkins J (2012) Reducing the ecological consequences of night-time light pollution: options and developments. J Appl Ecol 49:1256–1266. https://doi.org/10.1111/j.1365-2664.2012.02212.x
Gaston KJ, Visser ME, Hölker F (2015) The biological impacts of artificial light at night: the research challenge. Phil Trans R Soc B 370:20140133. https://doi.org/10.1098/rstb.2014.0133
Hopkins J, Baudry G, Candolin U, Kaitala A (2015) I’m sexy and I glow it: female ornamentation in a nocturnal capital breeder. Biol Lett 11:20150599. https://doi.org/10.1098/rsbl.2015.0599
Hopkins J, Baudry G, Lehtonen TK, Kaitala A (2021) Costly mating delays drive female ornamentation in a capital breeder. Ecol Evol 11:8863–8868. https://doi.org/10.1002/ece3.7719
Ineichen S, Rüttimann B (2012) Impact of artificial light on the distribution of the common European glow-worm, Lampyris noctiluca (Coleoptera: Lampyridae). Lampyrid 2:31–36
Kyba C, Mohar A, Posch T (2017) How Bright Is Moonlight. Astron Geophys 58:31–32
Lehtonen TK, Babic NL, Piepponen T, Valkeeniemi O, Borshagovski A-M, Kaitala A (2021) High road mortality during female-biased larval dispersal in an iconic beetle. Behav Ecol Sociobiol 75:26. https://doi.org/10.1007/s00265-020-02962-6
Lewis S (2016) Silent sparks: the wondrous world of fireflies. Princeton University Press, New Jersey
Lewis SM, Wong CH, Owens ACS, Fallon C, Jepsen S, Thancharoen A, Wu C, De Cock R, Novák M, López-Palafox T, Khoo V, Reed JM (2020) A global perspective on firefly extinction threats. Bioscience 70:157–167. https://doi.org/10.1093/biosci/biz157
Longcore T, Rich C (2016) Artificial night lighting and protected lands: ecological effects and management approaches. Natural Resource Report, Fort Collins, Colorado
Macgregor CJ, Pocock MJO, Fox R, Evans DM (2019) Effects of street lighting technologies on the success and quality of pollination in a nocturnally pollinated plant. Ecosphere 10:e02550. https://doi.org/10.1002/ecs2.2550
Owens ACS, Cochard P, Durrant J, Farnworth B, Perkin EK, Seymoure B (2020) Light pollution is a driver of insect declines. Biol Conserv 241:108259. https://doi.org/10.1016/j.biocon.2019.108259
Owens ACS, Lewis SM (2018) The impact of artificial light at night on nocturnal insects: a review and synthesis. Ecol Evol 8:11337–11358. https://doi.org/10.1002/ece3.4557
Owens ACS, Meyer-Rochow VB, Yang E-C (2018) Short- and mid-wavelength artificial light influences the flash signals of Aquatica ficta fireflies (Coleoptera: Lampyridae). PLoS One 13:1. https://doi.org/10.1371/journal.pone.0191576
Schwalb HH (1961) Beiträge zur Biologie der einheimischen Lampyriden Lampyris noctiluca und Phausis splendidula un experimental Analyse ihres Beutefang- und Sexualverhaltens. Zoologisches Jahrbuch 88:399–550
Sih A, Ferrari MC, Harris DJ (2011) Evolution and behavioural responses to human-induced rapid environmental change. Evol Appl 4:367–387. https://doi.org/10.1111/j.1752-4571.2010.00166.x
Slabbekoorn H (2013) Songs of the city: noise-dependent spectral plasticity in the acoustic phenotype of urban birds. Anim Behav 85:1089–1099. https://doi.org/10.1016/j.anbehav.2013.01.021
Spoelstra K, van Grunsven RHA, Donners M, Gienapp P, Huigens ME, Slaterus R, Berendse F, Visser ME, Veenendaal E (2015) Experimental illumination of natural habitat-an experimental set-up to assess the direct and indirect ecological consequences of artificial light of different spectral composition. Phil Trans R Soc B 370:20140129. https://doi.org/10.1098/rstb.2014.0129
Swaddle JP, Francis CD, Barber JR, Cooper CB, Kyba CC, Dominoni DM, Shannon G, Aschehoug E, Goodwin SE, Kawahara AY (2015) A framework to assess evolutionary responses to anthropogenic light and sound. Trends Ecol Evol 30:550–560. https://doi.org/10.1016/j.tree.2015.06.009
Therneau T (2020) A package for survival analysis in R. R package version 3.1–12. https://CRAN.R-project.org/package=survival. Accessed 6 May 2021
Tuomainen U, Candolin U (2011) Behavioural responses to human-induced environmental change. Biol Rev 86:640–657. https://doi.org/10.1111/j.1469-185X.2010.00164.x
Tyler J (2002) The Glow-worm. Lakeside Printing Ltd., Sevenoaks
Van den Broeck M, De Cock R, Van Dongen S, Matthysen E (2021a) Blinded by the light: artificial light lowers mate attraction success in female glow-worms (Lampyris noctiluca L.). Insects 12:734. https://doi.org/10.3390/insects12080734
Van den Broeck M, De Cock R, Van Dongen S, Matthysen E (2021b) White LED light intensity, but not colour temperature, interferes with mate-finding by glow-worm (Lampyris noctiluca L.) males. J Insect Conserv 25:339–347. https://doi.org/10.1007/s10841-021-00304-z
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Wong BBM, Candolin U (2015) Behavioral responses to changing environments. Behav Ecol 26:665–673. https://doi.org/10.1093/beheco/aru183
Acknowledgements
We thank Tvärminne Zoological Station for providing excellent facilities for data collection and two anonymous reviewers for helpful comments and suggestions.
Funding
Open access funding provided by University of Helsinki including Helsinki University Central Hospital. The work was funded by the Swedish Cultural Foundation in Finland (grant numbers 148370 to CE and 160603 to UC), the Maj and Tor Nessling Foundation (grant number 202000239 to CE), and the Academy of Finland (grant number 294664 to AK and TKL).
Author information
Authors and Affiliations
Contributions
Christina Elgert, Ulrika Candolin, and Arja Kaitala conceived the ideas and designed methodology; Christina Elgert collected the data; Christina Elgert and Topi K. Lehtonen analysed the data; Christina Elgert, Topi K. Lehtonen, and Ulrika Candolin wrote the first draft of the manuscript. All authors participated in writing of the manuscript and read and approved the final version of it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by J. C Choe.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the Topical Collection Using behavioral ecology to explore adaptive responses to anthropogenic change — Guest Editors: Jan Lindström, Constantino Macias Garcia, Caitlin Gabor
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elgert, C., Lehtonen, T.K., Kaitala, A. et al. The duration of artificial light defines sexual signalling in the common glow-worm. Behav Ecol Sociobiol 75, 154 (2021). https://doi.org/10.1007/s00265-021-03093-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-021-03093-2