Abstract
Males and females often have divergent evolutionary interests, generating sexual conflicts. This is particularly true in organisms that exhibit facultative sexuality, whereby females are capable of reproducing without fitness costs of mating. Here, we provide the first documented evidence with quantitative tracking showing that sex interacts with social context to determine space-use of females, in a pattern resembling predator avoidance. To achieve this, we labelled Daphnia magna with fluorescent nanoparticles and utilized a 3-D tracking platform to record pairs of individuals swimming. The recordings comprised either same-sex or opposite-sex pairings. We found that females swam faster, deeper, more horizontally, and more linearly when exposed to males than when exposed to females. Simultaneously, we found that male behavior did not differ depending on swimming partner and, importantly, we observed no sexual dimorphism in swimming behaviors when swimming with the same sex. Our results suggest that the presence of males in a population has the potential to influence the distribution of individuals, similarly to known threats, such as predation. This highlights that sexual conflict has clear spatial consequences and should be considered in such ecological frameworks, like the Landscape of Fear (LOF) concept. In a broader context, the connection of the evolutionary and social concept of sexual conflict and the ecological concept of LOF may improve our understanding of population dynamics and the spatial and temporal distribution of individuals in natural ecosystems.
Significance statement
Despite the wealth of studies that detail how predators affect their prey’s spatial behaviors, studies on the role of sex and social context on spatial behavior are rare. Addressing this dearth of information, we studied the swimming behaviors of an organism that can reproduce with or without sex, when exposed to an individual of either the same or opposite sex. We found no difference between the sexes in swimming behaviors; however, we revealed that females avoided males by swimming deeper in the water column, reminiscent of the response to predation. Our results highlight that social conflict between the sexes strongly affects the demographics of a population and may therefore have a substantial role in the spatial ecology of organisms in the wild.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A central challenge in ecological research is to understand the mechanisms behind differences in inter- and intraspecific spatial and temporal distributions of organisms. Interspecific differences are predominantly caused by competitive interactions or governed by predator distribution and behavior. For example, elk (Cervus elaphus) move from grassland to forested areas due to the presence of wolves (Canis lupus), or dugongs (Dugong dugon) utilize deeper waters to avoid predation from shallow water inhabiting tiger sharks (Galeocerdo cuvier) (Wirsing and Ripple 2011). With respect to intraspecific differences in distribution, sexual segregation is well-documented across many different taxa (Ludynia et al. 2013; Wang et al. 2018; Zak et al. 2020). Various causal mechanisms have been proposed, such as dimorphism creating different nutritional requirements, thereby segregating males and females outside the breeding season (Li et al. 2017), or avoidance of male harassment by females that have already mated (Ide 2011). Therefore, a general pattern may be that the intraspecific distribution of individuals might differ as a result of sexual conflict.
All taxa that reproduce sexually are likely to encounter some form of sexual conflict (Parker 1979), suggesting that the outcome of male–female interactions has divergent evolutionary optima for the two sexes (Chapman et al. 2003). Due to the impact of sexually antagonistic evolution, sexual conflict has been espoused as a major mechanism of evolution (Hosken and Snook 2005), with the ultimate consequences being the potential to lead to divergence within and between species (Parker and Partridge 1998; Gavrilets et al. 2001; Chapman 2018; Janicke et al. 2018). Such conflicts between the sexes can occur over various traits, including mating frequency, fertilization, and clutch size (Chapman et al. 2003). Generally, it is assumed that males benefit from maximizing such traits like mating frequency; however, females should favor lower mating rates due to the costs of sex (Gavrilets et al. 2001). These costs arise from many sources such as increased infection rates from contact with conspecifics (Thrall et al. 2000), fitness reducing seminal fluid accessory gland proteins introduced during copulation (Wigby and Chapman 2005), physical damage due to traumatic insemination (Stutt and Siva-Jothy 2001) or due to penile structures that prevent females escaping during copulation (Lange et al. 2013), increased energy demands (Nicol et al. 2019), and even increased predation risk (Magnhagen 1991).
One strategy females may employ to minimize such costs of mating is to avoid superfluous copulations such as if already mated. This is hypothesized to be more prevalent when the cost of mating is high and, therefore, females are more likely to be selective (Bleu et al. 2012). In most populations, irrespective of the fitness costs of mating, the requirement to mate in order to achieve any “fitness” may dampen the strength of these avoidance behaviors. In facultatively sexual populations however, females are potentially decoupled from the obligation to mate due to being able to reproduce asexually (Brewer 1998; Gerber and Kokko 2016). This may then lead to strong behavioral avoidance of males due to the potentially strong costs associated with mating. In such populations, it has been colorfully stated that the sequence of events in male behavior during reproductive attempts is fundamentally indistinguishable from predation attempts (Gerritsen and Strickler 1977; Brewer 1998). If true, this suggests that the male poses a potential risk to fitness for the female and consequently, the demographics of a population may have a substantial role in the spatial ecology of the population.
Daphnia magna is a common facultatively sexual freshwater cladoceran. Predominantly, Daphnia reproduce asexually but under certain suboptimal environmental conditions, such as at high population densities, they often switch to a sexual reproductive phase (Kleiven et al. 1992; Haltiner et al. 2020). This switch means that they produce a maximum of 2 genetically non-identical eggs through recombination, instead of bearing an asexual clutch of up to 110 live clones (Gerber et al. 2018). Change in reproductive mode is not a one-way street, and females can continue to alternate strategies between broods. Therefore, females may be under strong pressure to avoid potentially costly mating events if in the asexual phase or already mated, and could conceivably adopt different swimming behaviors as a proactive avoidance measure. Multiple studies have investigated how swimming behaviors differ between sexes of many cladocerans (D. pulicaria (Brewer 1998); D. obtusa (La et al. 2014); Polyphemus pediculus (Butorina 2000); Chydorus sphaericus (Van Damme and Dumont 2006)); however, most studies investigate interactions between the sexes, and therefore focus on group behavior or the mating behavior in high density environments. To the best of our knowledge, no studies have investigated how changes in the sex ratios affect an individual’s swimming behavior, leading to that our understanding of sexual conflicts in a spatial context is still elusive.
Hence, the purpose of our study is to disentangle the individual behavioral responses of D. magna in the presence of conspecifics. We hypothesize that the potential reduction in fitness due to sexual reproduction will cause females to display avoidance behaviors when paired with males. Using 3-D tracking of individual animals, we were able to quantify the speed at which males and females swim, their average swimming depth, and the tortuosity (or the linearity) of their swimming paths, i.e., we were able to map the individual behavior in different social contexts.
Specifically, we expect that due to the sexual dimorphism in size (Mitchell 2001), females will swim faster than males, and when swimming with the opposite sex, this speed will increase. Similarly, due to depth serving as a refuge from many threats, such as predation and ultraviolet radiation (UVR) (Hansson and Hylander 2009b; Ekvall et al. 2015, 2020), we predict that females exposed to males will swim deeper in the water column than either males or females swimming with females. Multiple observations of zooplankton have described male “scanning” behavior which involves males swimming more horizontally than vertically in a bid to increase encounter chance with a female (Gerritsen 1980; Brewer 1998). Therefore, we predict that males will swim more linearly and more horizontally than females, with this effect being more pronounced when swimming with the opposite sex. In short, using 3-D tracking, we are able to provide the first insights into how sexual conflict interacts with an individual’s social context, thereby causing spatial variation in swimming behavior.
Methodology
Culture conditions
D. magna used in this experiment were isolated from a laboratory culture on the 12 August 2019, which was originally inoculated with several genotypes from a population in Lake Bysjön (55.6753 lat, 13.5452 long) in southern Sweden. They were maintained at high densities in a 400-L plastic mesocosm at 20 °C with a 16 h:8 h light:dark photoperiod and routinely fed with a predominantly Tetradesmus obliquus (formerly Scenedesmus obliquus) algal suspension. Once isolated, D. magna were sexed using a stereomicroscope (Olympus SZX7, Japan). Males were identified by three characteristic morphological traits: (1) the smaller and rounder rostrum in comparison to females, (2) the elongated and motile antennules, and (3) the pronounced hook at the end of the first leg (Mitchell 2001). Females were identified by the absence of these traits. The sex ratio of the initial population was approximately 10:1 (female:male). After being sexed, D. magna were maintained in single sex populations of 30 individuals per liter at the same temperature and photoperiod as the source population and fed with the same algal suspension ad libitum until being assayed. The behavioral assays took place over the period of 21–23 August 2019, that is, individuals were only isolated within their own sex for between 9 and 11 days; therefore, later exposure to the opposite sex was not a novel experience.
Experimental design
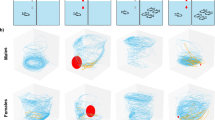
In order to determine swimming behaviors of individual Daphnia, we used a proven protocol (Ekvall et al. 2013; Palmér et al. 2016; Langer et al. 2019). This required labelling each individual with either red or yellow nanoparticles (Qdot™ ITK™ Carboxyl Quantum Dots; Life Technologies Corporation, USA) that fluoresce when excited by blue light (465 nm). This allowed us to identify both recorded individuals simultaneously. The labelling process involved binding the quantum dots to the carapace of the organism by incubating individual Daphnia in 2-ml centrifuge tubes with 250-µl aged tap water and 33-µl quantum dot labelling solution for 1 h in the absence of light, before removing the excess solution by rinsing the organism with aged tap water (Langer et al. 2019). Once the Daphnia were labelled, they were recorded in a Plexiglas aquarium (0.2 × 0.2 × 0.75 m; filled with only aged tap water) with four cameras (Pike F-210C color cameras, Allied Vision Technologies GmbH) positioned as vertical stacked stereopairs towards the aquarium to allow 3-dimensional positioning to be acquired, and the only light source was a lighting array above supplying blue light–emitting diodes with peak emission at 465 nm (VANQ Technology). The surface light intensity was 223.4 μmol m−2 s−1 at the top and 78.2 μmol m−2 s−1 at the bottom (see Langer et al. 2019) which has previously been equated to a night-like condition (Ekvall et al. 2020). To discern the effects of conspecific sex on an individual’s swimming behavior, the Daphnia were recorded in pairs. This produced three sex combinations or “treatments”: females recorded with females (n = 19), males with males (n = 20) and females with males (n = 22). The pairs of individuals were obtained from separate holding aquaria (see above), and introduced to the tracking aquarium simultaneously. They were given 1 min of acclimatization before the 3-min recording began. This setup allowed us to extract multiple metrics of swimming behavior, such as the individual’s speed, depth, horizontal direction changes (horizontal net displacement ratio [HNDR]), and the tortuosity of their swimming path (net gross displacement ratio [NGDR]), as well as calculating the distance between individuals. The water was then replaced between trials to prevent lingering chemical cues influencing subsequent recordings. Due to the nature of the experiment, blinding was not possible.
Data handling and statistical analysis
We used a custom-built MATLAB application (Palmér et al. 2016; The MathWorks, Inc. 2017) to extract the Daphnia’s 3D positions from the recordings. Using the 3D coordinates, depth was extracted as Z coordinates, and speed was calculated as the gross distance travelled every second. HNDR was calculated as the ratio of horizontal distance travelled to the gross distance travelled every second. Similarly, the NGDR was calculated as the ratio of net distance travelled to the gross distance travelled every second. Therefore, for either ratio, the more vertical or indirect the swimming path, the lower the ratios, and conversely, the more horizontal or linear the path, the higher the HNDR and NGDR, respectively. Due to the recording frame rate (6 fps) producing between 360 and 1080 points per variable, all variables were averaged using the median values as to limit the influence of extreme values. Henceforth, all “averages” discussed refer to the median. Statistical analysis was subsequently conducted in R version R v3.6.2 (R Core Team 2019). Figures were also drawn with R v3.6.2 utilizing the package “ggplot2” (Wickham 2016). The data and code for this study are archived online (Lee et al. 2021a, b).
To examine the effects of sex and conspecific sex on speed, we performed a linear mixed model using the package “nlme” (Pinheiro et al. 2021). Average speed served as the dependent variable, and “sex,” “conspecific sex,” and their interaction were used as fixed effects with the recording ID serving as a random effect to account for the non-independence of recording individuals in pairs. Size was also included as a covariate for the analysis of average speed, due to larger individuals having the potential to swim faster (Dodson and Ramcharan 1991; Hylander et al. 2014). Due to the physical constraints of the experimental arena, we treated the average depth as a ratio of vertical position for the analysis, i.e., occupied depth in relation to of the total available depth. As depth, horizontal movements (HNDR), and tortuosity (NGDR) variables represent ratios derived from continuous numbers, we employed beta regression mixed models (Douma and Weedon 2019). “Sex,” “conspecific sex,” and their interactions were also used in these models as fixed effects with the recording ID as a random effect. All models were graphically investigated, and the examination of significant differences in main effects was conducted using the post hoc Tukey’s test. In the case of the dependent variable depth, we note that the model appears over-dispersed; however, other forms of modelling provide less accuracy due to the methodological design. To provide easier interpretation of the results, we back transformed the depth variable to show actual depth as opposed to a ratio of vertical position.
Results
Speed
Swimming speed was, as expected, influenced by size with larger individuals swimming faster than smaller ones (Table 1; online resource Fig. S1.). Yet, when accounting for size, we found no single effect of sex on swimming speed. There was, however, an effect of swimming partner on the speed, as different sex pairs show distinct speeds, while same-sex pairs show no difference. Importantly, we observed a strong interaction between sex and the swimming partner. This effect was entirely driven by the females swimming faster when swimming with a male than with a female (Tukey’s test; p = 0.003). Specifically, females from same-sex pairs swam approximately 32% slower than females swimming with the opposite sex (Fig. 1a). Males did not adjust swimming speed with different swimming partners (Tukey’s Test; p = 0.93) swimming at 13.81 mm s−1 (± 1.14 SE) with females and 13.05 mm s−1 (± 1.08 SE) with other males.
Effects of sex and conspecific sex on various metrics of swimming behavior in Daphnia magna. The subplots refer to (a) average speed considering size as a covariate, (b) average depth, (c) average ratio of horizontal to vertical movements (HNDR) with 1 being completely horizontal and 0 being completely vertical, and (d) the average net to gross displacement ratio (NGDR) with higher values indicating a lower turning rate. All subplots show the model estimates ± 2 SE with raw values (individuals) plotted as faded points. The dashed lines in subplot (b) represent the boundaries of the water column
Depth
Swimming partner also influenced the focal individual’s swimming depth whereby same-sex pairs swam higher in the water column than opposite sex pairs (Table 2). There was also evidence that males preferred swimming higher in the water column than females. Similar to speed, there was a clear interaction effect of sex and swimming partners with females having an estimated mean swimming depth of 586 mm (± 38 SE) when swimming with a male, whereas swimming with another female averaged 379 mm (± 39 SE) (Tukey’s test; p = < 0.001), that is, females swam 64% deeper in the presence of a male than of a female (Fig. 1b). Males however had similar depth preferences irrespective of the swimming partner’s sex (Tukey’s Test; p = 0.97).
HNDR
Horizontal movements (HNDR) did not conform to prior expectations, since males swimming with females were less likely to perform horizontal movements than their female counterparts (Fig. 1c). The sex of the swimming partner also had a notable impact on the ratio of horizontal movements, with females in same-sex pairs being less likely to perform horizontal movements than with the opposite sex. The interaction of sex and swimming partner sex also emerged as significant. This appears to be driven by females paired with males, as they were 15% more likely to swim horizontally than their male counterparts, or 11% more likely to swim horizontally than females exposed to a female (Tukey’s tests; p < 0.001 and p = 0.004 respectively). Males in comparison did not differ in HNDR when exposed to a female or to a male (Tukey’s test; p = 0.99).
NGDR
The tortuosity (NGDR) of an individual’s swimming path showed considerable influence of all main fixed effects. Female Daphnia exposed to a male were more likely to have linear swimming paths than their male partners (Fig. 1d), and female same-sex pairs were also more likely to swim more linearly than females with males. The interaction between both sex and the sex of the swimming partner yielded an effect, which once again was solely driven by females paired with males. Females were approximately 15% more likely to swim linearly than their male counterparts (Tukey’s test; p < 0.001).
Discussion
Sexual conflict is a widespread social phenomenon among sexually reproducing organisms with the potential to shape macroevolutionary patterns (Bonduriansky 2011). Despite the prevalence of obligately sexual organisms, there are numerous examples of alternative reproduction strategies, including facultative sexuality (Burke and Bonduriansky 2017; Kobayashi 2019) among, for example, numerous common and globally distributed organisms, such as many crustacean taxa. Here, we show that facultatively sexual D. magna females modify their behavior when exposed to males, swimming faster, deeper, more horizontally, and straighter than when exposed to other females. In contrast, males do not alter swimming behavior depending on their swimming partners’ sex. This finding suggests that the costs of sexual reproduction for females trigger their avoidance of conspecific males.
Past studies of the genus Daphnia have frequently looked at mating behaviors, and generally focused on how males increase their probability of encountering a female (Brewer 1998), neglecting how females act in these situations. This is often a consequence of the methodological design, which requires both males and females to be present in order to observe potential mating events. However, in nature, there are often times when males will be absent and curiously, few studies use single sex populations as controls, and yet extract and discuss the behavior of females (Brewer 1998). Here, we provide this missing information and observe single sex and opposite sex pairings.
In several studies, it has been discussed that D. pulicaria males swim twice as fast as females in a bid to increase encounter rates via “scanning” behaviors similar to other zooplankton taxa (Brewer 1998). The scanning behaviors are characterized by more horizontal and linear movements (Gerritsen 1980), and have even been suggested to be widespread in the planktonic community as they are found in copepods from both marine and freshwater environments, as well as in Daphnia species (Gerritsen 1980; La et al. 2014). Intuitively, this appears logical as, if males can exploit a single plane with relatively straight movements, it increases the probability of encountering randomly distributed resources, such as females (Dusenbery 1992; Visser 2007). D. magna, however, do not appear to echo this pattern as, when accounting for size, we show here that males do not swim faster than females, and only females alter their speed according to their swimming partners. Moreover, we observed that females appear more likely to perform more horizontal and straighter movements when exposed to males, although we cannot exclude that this may be an artefact since females cannot swim further in the vertical plane when reaching the bottom and were therefore forced to swim more horizontally. Despite this, males do not appear to differ from females when in same-sex pairings nor when paired with a female, which supports our view that this “scanning” behavior does not occur in D. magna and calls into question whether this is indeed a widespread behavior in zooplankton (Brewer 1998). Instead, our results give credence to the notion that males’ likelihood of reproducing relies heavily on chance encounters with sexually responsive females (Kawatsu 2013; Gerber and Kokko 2016).
Despite the lack of similarity in the previous swimming behaviors recorded with other species, the use of depth as a refuge is well described for D. magna. They use deeper waters to attenuate harmful ultraviolet radiation (UVR) and avoid predation from visually hunting predators, such as fish (Hansson and Hylander 2009b). Similarly, our data show that female Daphnia respond to males in a pattern resembling a threat response (online resource Fig. S2), i.e., the female avoids the male by diving deeper. Despite the clear overall response, not all females resided deeper in the water column. This variation in optimal depth may be a result of sexual receptivity, although we cannot rule out other factors such as genetic variation or energetic state which likely contribute to this trait. Interestingly, males did not display a propensity to follow the majority of females to the deeper waters, which strengthens findings from previous studies showing males to only be able to follow females for a few body lengths (Brewer 1998); however, to what degree this is male sensory limitations or choice in pursuit is unknown. Furthermore, we did not observe any explicit following of partners in any treatment group when looking at the distance between individuals (data not shown).
The consequences of the observed avoidance may have far-reaching effects on the population dynamics. For instance, the energetic cost of performing avoidance behaviors has been demonstrated to reduce population growth in a facultatively sexual invertebrate (Nelson 2007). Also, male Daphnia in particular have been shown to be more positively phototactic than females (De Meester 1993), which suggests they inhabit higher strata than females, which may thereby explain the female use of depth as a refuge, as shown in our study. However, for the females inhabiting deeper waters, there are further potential metabolic costs. For example, if in a sufficiently deep lake, the temperature gradient in the deeper waters may reduce metabolic rates (Dawidowicz and Loose 1992), coupled with lower food availability and quality, which suggests that population growth would be retarded. That said, it is well established that D. magna perform diel vertical migration (DVM) as a foraging strategy to avoid the higher predation risk and UVR during the day, foraging in the food-rich surface waters during the night (Hansson and Hylander 2009a, b). This strategy could mitigate some of the potential costs of males inhabiting the higher strata, especially if mating success is increased with light availability. Under this scenario, our results suggest that sexual conflict could be a further selective pressure contributing to the evolution of this DVM behavior.
In accordance with our findings, the sequence of events by males during reproductive attempts has previously been mentioned as being indistinguishable from predation attempts (Gerritsen and Strickler 1977; Brewer 1998). Predator–prey interactions in a spatially explicit context have been extensively studied (Miller et al. 2014; Fortin et al. 2015; Schmitz et al. 2017), whereas the spatial ecology of sexual conflict has received relatively little attention. An emergent framework within the predator–prey domain aimed at clarifying and refocusing the effort to understand the spatial effects of risk is the “Landscape of Fear” (LOF) (Laundré et al. 2001, 2010, 2014; Brown and Kotler 2004; Gaynor et al. 2019). The LOF has been defined as the spatial variation in prey perception of predation risk. In order to proactively minimize such risks, the LOF concept suggests that two behavioral strategies may be employed, and they are (1) avoiding areas of high predation risk and (2) modifying behavior in a location to reduce the probability of predation (Gaynor et al. 2019). Replacing the word “predation” with “mating,” we see that Daphnia do indeed avoid areas of high risk, i.e., where males are located. Therefore, based on the potential for sexual conflict in this facultatively sexual species (Gerber and Kokko 2016), we suggest that wherever there are probable fitness costs, this framework could be applied. Our results highlight that demographic features such as reproductive mode or sex ratios which vary over the season may be an important factor in the perception of risk for female individuals and is an avenue for further research.
In conclusion, we observe here that males and females of D. magna lack sexual dimorphism in swimming behaviors. However, when in the presence of the opposite sex, females demonstrate behaviors consistent with strong male avoidance, leading to a skewed depth distribution among sexes. These avoidance behaviors are analogous to other threat responses, such as to predation risk or ultraviolet radiation, which have been shown to have fitness consequences. Therefore, we advocate that incorporating predominantly evolutionary concepts, such as sexual conflict, to the ecological frameworks, like the Landscape of Fear, has the potential to improve our understanding of the mechanisms determining the distributions of individuals in space and time.
Data availability
Data supporting the results are available online in the Dryad data repository at https://doi.org/10.5061/dryad.2ngf1vhm8.
Code availability
The code used to analyze the data is available online in the Zenodo repository at http://doi.org/10.5281/zenodo.5112715.
Change history
04 November 2021
Insertion of funding note to fulfill the contractual requirement of the Compact agreement.
References
Bleu J, Bessa-Gomes C, Laloi D (2012) Evolution of female choosiness and mating frequency: effects of mating cost, density and sex ratio. Anim Behav 83:131–136
Bonduriansky R (2011) Sexual selection and conflict as engines of ecological diversification. Am Nat 178:729–745
Brewer MC (1998) Mating behaviours of Daphnia pulicaria, a cyclic parthenogen: comparisons with copepods. Philos T Roy Soc B 353:805–815
Brown JS, Kotler BP (2004) Hazardous duty pay and the foraging cost of predation. Ecol Lett 7:999–1014
Burke NW, Bonduriansky R (2017) Sexual conflict, facultative asexuality, and the true paradox of sex. Trends Ecol Evol 32:646–652
Butorina LG (2000) A review of the reproductive behavior of Polyphemus pediculus (L.) Muller (Crustacea : Branchiopoda). Hydrobiologia 427:13–26
Chapman T (2018) Sexual conflict: mechanisms and emerging themes in resistance biology. Am Nat 192:217–229
Chapman T, Arnqvist G, Bangham J, Rowe L (2003) Sexual conflict. Trends Ecol Evol 18:41–47
Dawidowicz P, Loose CJ (1992) Cost of swimming by Daphnia during diel vertical migration. Limnol Oceanogr 37:665–669
De Meester L (1993) Inbreeding and outbreeding depression in Daphnia. Oecologia 96:80–84
Decaestecker E, De Meester L, Mergeay J (2009) Cyclical Parthenogenesis in Daphnia: sexual versus asexual reproduction. In: Schön I, Martens K, Dijk P (eds) Lost Sex: The Evolutionary Biology of Parthenogenesis. Springer, Netherlands, Dordrecht, pp 295–316
Dodson S, Ramcharan C (1991) Size-specific swimming behavior of Daphnia pulex. J Plankton Res 13:1367–1379
Douma JC, Weedon JT (2019) Analysing continuous proportions in ecology and evolution: a practical introduction to beta and Dirichlet regression. Methods Ecol Evol 10:1412–1430
Dusenbery DB (1992) Sensory ecology. Freeman, New York
Ekvall MT, Bianco G, Linse S, Linke H, Bäckman J, Hansson L-A (2013) Three-dimensional tracking of small aquatic organisms using fluorescent nanoparticles. PLoS ONE 8:8
Ekvall MT, Hylander S, Walles T, Yang X, Hansson L-A (2015) Diel vertical migration, size distribution and photoprotection in zooplankton as response to UV-A radiation. Limnol Oceanogr 60:2048–2058
Ekvall MT, Sha Y, Palmér T, Bianco G, Bäckman J, Åström K, Hansson L-A (2020) Behavioural responses to co-occurring threats of predation and ultraviolet radiation in Daphnia. Freshwater Biol 65:1509–1517
Fortin D, Buono PL, Schmitz OJ, Courbin N, Losier C, St-Laurent MH, Drapeau P, Heppell S, Dussault C, Brodeur V, Mainguy J (2015) A spatial theory for characterizing predator - multiprey interactions in heterogeneous landscapes. Proc R Soc B 282:99–108
Gavrilets S, Arnqvist G, Friberg U (2001) The evolution of female mate choice by sexual conflict. Proc R Soc B 268:531–539
Gaynor KM, Brown JS, Middleton AD, Power ME, Brashares JS (2019) Landscapes of fear: spatial patterns of risk perception and response. Trends Ecol Evol 34:355–368
Gerber N, Kokko H (2016) Sexual conflict and the evolution of asexuality at low population densities. Proc R Soc B 283:20161280
Gerber N, Kokko H, Ebert D, Booksmythe I (2018) Daphnia invest in sexual reproduction when its relative costs are reduced. Proc R Soc B 285:20172176
Gerritsen J (1980) Sex and parthenogenesis in sparse populations. Am Nat 115:718–742
Gerritsen J, Strickler JR (1977) Encounter probabilities and community structure in zooplankton - mathematical-model. J Fish Res Board Can 34:73–82
Haltiner L, Hänggi C, Spaak P, Dennis SR (2020) Sex in crowded places: population density regulates reproductive strategy. Hydrobiologia 847:1727–1738
Hansson L-A, Hylander S (2009a) Effects of ultraviolet radiation on pigmentation, photoenzymatic repair, behavior, and community ecology of zooplankton. Photochem Photobio S 8:1266–1275
Hansson L-A, Hylander S (2009b) Size-structured risk assessments govern Daphnia migration. Proc R Soc B 276:331–336
Hosken D, Snook R (2005) How important is sexual conflict? Am Nat 165:S1–S4
Hylander S, Ekvall MT, Bianco G, Yang X, Hansson L-A (2014) Induced tolerance expressed as relaxed behavioural threat response in millimetre-sized aquatic organisms. Proc R Soc B 281:7
Ide JY (2011) Avoiding Male Harassment: Wing-Closing Reactions to Flying Individuals by Female Small Copper Butterflies. Ethology 117:630–637
Janicke T, Ritchie MG, Morrow EH, Marie-Orleach L (2018) Sexual selection predicts species richness across the animal kingdom. Proc R Soc B 285:20180173
Kawatsu K (2013) Sexual conflict over the maintenance of sex: effects of sexually antagonistic coevolution for reproductive isolation of parthenogenesis. Plos One 8(2):e58141
Kleiven OT, Larsson P, Hobaek A (1992) Sexual reproduction in Daphnia magna requires 3 stimuli. Oikos 65:197–206
Kobayashi K (2019) Sexual reproduction and diversity: connection between sexual selection and biological communities via population dynamics. Popul Ecol 61:135–140
La GH, Choi JY, Chang KH, Jang MH, Joo GJ, Kim HW (2014) Mating behavior of Daphnia: impacts of predation risk, food quantity, and reproductive phase of females. Plos One 9(8):e104545
Lampert W, Lampert KP, Larsson P (2012) Induction of male production in clones of Daphnia pulex by the juvenoid hormone methyl farnesoate under short photoperiod. Comp Biochem Phys C 156:130–133
Lange R, Reinhardt K, Michiels NK, Anthes N (2013) Functions, diversity, and evolution of traumatic mating. Biol Rev 88:585–601
Langer SM, Weiss LC, Ekvall MT, Bianco G, Hansson L-A, Tollrian R (2019) A three-dimensional perspective of Daphnia’s swimming behavior with and without predator cues. Limnol Oceanogr 64:1515–1525
Laundré JW, Hernandez L, Altendorf KB (2001) Wolves, elk, and bison: reestablishing the “landscape of fear” in Yellowstone National Park, USA. Can J Zoo 79:1401–1409
Laundré JW, Hernandez L, Medina PL, Campanella A, Lopez-Portillo J, Gonzalez-Romero A, Grajales-Tam KM, Burke AM, Gronemeyer P, Browning DM (2014) The landscape of fear: the missing link to understand top-down and bottom-up controls of prey abundance? Ecology 95:1141–1152
Laundré JW, Hernández L, Ripple WJ (2010) The landscape of fear: ecological implications of being afraid. The Open Ecol J 3:1–7
Lee M, Solano Udina C, Hansson L-A (2021a) Data supporting fear of sex: sexual conflict exposed as avoidance in a parthenogenetic invertebrate. Dryad, Dataset. https://doi.org/10.5061/dryad.2ngf1vhm8
Lee M, Solano Udina C, Hansson L-A (2021b) Data supporting fear of sex: sexual conflict exposed as avoidance in a parthenogenetic invertebrate. Zenodo. https://doi.org/10.5281/zenodo.5112716
Li B, Wang MY, Blank DA, Xu WX, Yang WK, Ruckstuhl KE (2017) Sexual segregation in the Darwin’s wild sheep, Ovis ammon darwini, (Bovidae, Artiodactyla), in the Mengluoke Mountains of Xinjiang, China. Folia Zool 66:126–132
Ludynia K, Dehnhard N, Poisbleau M, Demongin L, Masello JF, Voigt CC, Quillfeldt P (2013) Sexual segregation in rockhopper penguins during incubation. Anim Behav 85:255–267
Magnhagen C (1991) Predation risk as a cost of reproduction. Trends Ecol Evol 6:183–185
Miller JRB, Ament JM, Schmitz OJ (2014) Fear on the move: predator hunting mode predicts variation in prey mortality and plasticity in prey spatial response. J Anim Ecol 83:214–222
Mitchell SE (2001) Intersex and male development in Daphnia magna. Hydrobiologia 442:145–156
Nelson EH (2007) Predator avoidance behavior in the pea aphid: costs, frequency, and population consequences. Oecologia 151:22–32
Nicol SC, Morrow GE, Harris RL (2019) Energetics meets sexual conflict: the phenology of hibernation in Tasmanian echidnas. Funct Ecol 33:2150–2160
Palmér T, Bianco G, Ekvall MT, Hansson L-A, Åström K (2016) Calibration, positioning and tracking in a refractive and reflective scene. In: 23rd international conference on pattern recognition (ICPR). IEEE, pp 3810–3815
Parker GA (1979) Sexual selection and sexual conflict. In: Blum MS, Blum NA (eds) Sexual selection and reproductive competition in insects. Academic Press, New York, pp 123–166
Parker GA, Partridge L (1998) Sexual conflict and speciation. Philos T Roy Soc B 353:261–274
Pinheiro J, Bates D, DebRoy S, Sarkar D (2021) nlme: linear and nonlinear mixed effects models. R package version 3.1-152. https://CRAN.R-project.org/package=nlme
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed 12 Dec 2019
Schmitz OJ, Miller JRB, Trainor AM, Abrahms B (2017) Toward a community ecology of landscapes: predicting multiple predator-prey interactions across geographic space. Ecology 98:2281–2292
Slusarczyk M (1995) Predator-induced diapause in Daphnia. Ecology 76:1008–1013
Stutt AD, Siva-Jothy MT (2001) Traumatic insemination and sexual conflict in the bed bug Cimex lectularius. P Natl Acad Sci USA 98:5683–5687
The MathWorks, Inc. (2017) MATLAB release 2017b. In: Natick, Massachusetts, United States. https://se.mathworks.com/products/matlab.html
Thrall PH, Antonovics J, Dobson AP (2000) Sexually transmitted diseases in polygynous mating systems: prevalence and impact on reproductive success. Proc R Soc B 267:1555–1563
Van Damme K, Dumont HJ (2006) Sex in a cyclical parthenogen: mating behaviour of Chydorus sphaericus (Crustacea; Branchiopoda; Anomopoda). Freshwater Biol 51:2334–2346
Visser AW (2007) Motility of zooplankton: fitness, foraging and predation. J Plankton Res 29:447–461
Wang MY, Alves J, da Silva AA, Yang WK, Ruckstuhl KE (2018) The effect of male age on patterns of sexual segregation in Siberian ibex. Sci Rep 8:9
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Wigby S, Chapman T (2005) Sex peptide causes mating costs in female Drosophila melanogaster. Curr Biol 15:316–321
Wirsing AJ, Ripple WJ (2011) A comparison of shark and wolf research reveals similar behavioral responses by prey. Front Ecol Environ 9:335–341
Zak J, Prchalova M, Smejkal M, Blabolil P, Vasek M, Matena J, Riha M, Peterka J, Sed’a J, Kubecka J (2020) Sexual segregation in European cyprinids: consequence of response to predation risk influenced by sexual size dimorphism. Hydrobiologia 847:1439–1451
Acknowledgements
We would like to thank Giuseppe Bianco and Charlie Cornwallis for helpful discussions. We would also like to thank Kevin Jones for experimental assistance.
Funding
Open access funding provided by Lund University. Financial support was provided by the Swedish Research Council (VR) grant # 2016–03552 and the Royal Physiographic Society.
Author information
Authors and Affiliations
Contributions
All authors conceived the project and contributed to writing. CSU and ML collected data. ML performed the data analysis and wrote the first version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All animal handling and husbandry was conducted in accordance with approved institutional guidelines. The license M182-15 was granted by the Malmö/Lund authority for ethics of animal experimentation.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by D. J Hosken.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marcus Lee and Carlota Solano Udina contributed equally
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, M., Solano Udina, C. & Hansson, LA. Fear of sex: sexual conflict exposed as avoidance in a parthenogenetic invertebrate. Behav Ecol Sociobiol 75, 115 (2021). https://doi.org/10.1007/s00265-021-03054-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-021-03054-9