Abstract
Climate change and the subsequent increase of global temperature are the most current and important threats to biodiversity. Despite the importance of temperature, our knowledge about the level of behavioural and physiological adaptations of ant species from temperate regions to cope with high temperatures is limited compared to the broad knowledge of typical thermal specialists from warmer regions. In the current study, we investigated the temperature-related foraging risk of xerothermic ant species from the temperate climate in Europe, Formica cinerea. Our aims were to check how an increase in external soil temperature affects the foraging activity of workers and how the temperature during development and worker age affects foraging activity in high temperatures. Based on our results, we can draw the following conclusions: (1) the majority of workers utilize a risk-aversive strategy in relation to foraging in high surface temperatures; (2) pupal development temperature affects the risk taken by adult workers: workers that developed in a higher temperature forage more often but for shorter intervals compared to workers that developed in a lower temperature; (3) age is an important factor in temperature-related foraging activity, as with increasing age, workers forage significantly longer at the highest temperatures. Our study is one of the first to assess the potential factors that can affect the foraging risk of ants from a temperate climate in high ambient temperatures.
Significance statement
Our study is the first direct test of workers' age and the development temperature of pupae on the thermal-related foraging strategy of adult F. cinerea workers. It shows that worker age and the development temperature of pupae interact to promote tolerance of thermal stress. We found that with increasing age, workers are prone to forage significantly longer at the highest and riskiest temperatures. Workers that developed in the high temperature (28°C) foraged more often but for shorter intervals compared to workers that developed in the lower temperature (20°C). Interestingly, the factor of age is more significant for ants that developed in the higher temperature of 28°C; the foraging time of these ants significantly increased with their age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is among the most current and important threats to biodiversity due to the global increase of temperature. It can affect the life of organisms at different levels, from altering the life history traits of single organisms (e.g. Piantoni et al. 2019) to shifting species distribution patterns (e.g. Bellard et al. 2012). The earth’s surface temperature has increased by 0.6 °C during the nineteenth century and this is likely to reach 1.5 °C between 2030 and 2052 compared to the pre-industrial level (IPCC 2018). Increasing global temperature has a profound effect on the functioning of biological systems, e.g. it advances the timing of spring events (earlier arrival and breeding of migrant birds, earlier appearance of butterflies, earlier spawning of amphibians or plant flowering), shifts species distribution poleward in latitude and upward in elevation, alters community composition and could result in the widespread phenological desynchronization among species (Walther et al. 2002; Parmesan and Yohe 2003; Thackeray et al. 2016; Wersebe et al. 2019). The importance of temperature was neatly emphasised in this sentence: ‘unlike many other variables that concern biologists, the temperature is not just a property of life; it is a property of matter. Nothing escapes its control’ (Angilletta 2009).
Temperature strongly influences the foraging activity of ectothermic animals, such as ants, as high surface temperatures are correlated with increasing risks of desiccation or heat stress (e.g. Cerdá et al. 1998; Chung and Lin 2017; Friedman et al. 2019; Perez and Aron 2020). There are some species among ants that are well adapted to live in habitats with extremely high temperatures, such as deserts or sand dunes (Cerdá and Retana 2000; Cerdá 2001; Boulay et al. 2017). They exhibit a number of adaptations to live and survive in such difficult conditions (Wehner et al. 1992; Christian and Morton 1992; Gehring and Wehner 1995; Cerdá and Retana 2000; Cerdá 2001; Evgen’ev et al. 2007; Clémencet et al. 2010; Schultheiss and Nooten 2013), but even for them, thermal stress is a major cost of foraging (Merkt and Taylor 1994; Angilletta 2009; Ślipiński 2017). Still, some desert ant species forage when the surface temperature exceeds 50 °C, e.g. Pogonomyrmex californicus (Bernstein 1974, 1979), and even at the extreme level of 60 °C, as is the case for Cataglyphis spp. (Wehner et al. 1992). For ants living in such habitats, risk can be perceived from the perspective of critical physiological thermal limits: workers may utilize a risk-prone strategy (when foraging close to the critical thermal limit) or risk-aversive strategy (far from the critical thermal limit) when foraging for food (see Cerdá et al. 1998; Cerdá and Retana 2000; Cerdá 2001).
We still know little about the proximate mechanisms that determine and maintain the inter-individual behavioural variability of social insects, and the extent to which environmental factors can shape this behavioural variation (Tautz et al. 2003; Jones et al. 2005; Jeanson and Weidenmüller 2014). For example, it was demonstrated that the temperature experienced during larval development affects the learning abilities and recruitment behaviour of the honey bee (Tautz et al. 2003; Jones et al. 2005) or it can modify the response thresholds and temperature preferences of adult brood-tending Camponotus rufipes ant workers (Weidenmüller et al. 2009). Another study showed that higher development temperature (28 °C and 32 °C vs 24 °C) influenced positively the thermoresistance of Aphenogaster senilis workers (Oms et al. 2017). Differences in thermal tolerance observed among populations of thermophilic A. iberica could also be the result of acclimation during development (Villalta et al. 2020).
In the temperate climate, extremely thermally specialized ant species, such as some of the genus Cataglyphis, are rare and they are mostly limited to the southern regions due to the different environmental conditions. Dunes or sun-exposed sandy patches in temperate regions differ from typical deserts; therefore, the biology of xerothermic species inhabiting them (e.g. Formica cinerea) also differs. Nonetheless, in particular circumstances, the conditions in both types of habitats may temporarily become very similar in terms of extremely high surface temperatures. Therefore, studying the thermal response of species such as F. cinerea may provide an opportunity to determine the mechanisms and behaviours behind the adaptations of temperate zone species to cope with high surface temperatures, especially in the circumstances of inevitable global warming. Formica cinerea may be a good model species in estimating the effect of climate change on ectothermic animals because we know that typical heat tolerant species seems to be already at the edge of their physiological and behavioural capabilities (Andrew et al. 2013). However, we still know little about the potential thermal adaptations of species from temperate regions.
In the current study, we investigated the temperature-related foraging risk of the xerothermic ant species F. cinerea. More particularly, we asked: (1) how the increase of external temperature affects the foraging activity of workers and (2) how the temperature during pupal development and worker age affects foraging activity in high temperatures. Based on our previous findings (Ślipiński et al. 2015; Ślipiński 2017), we hypothesized that an increased external temperature has an effect on the foraging activity of this species, but also there is an individual behavioural variation in foraging risks taken by the foragers of this species—some workers take more risks than others. Based on literature data, we assumed that one of the factors influencing foraging strategies may be development temperature (Crill et al. 1996; Weidenmüller et al. 2009; Oms et al. 2017; Villalta et al 2020). We expected that workers developing in higher temperatures could be more heat resistant and willing to take more thermal risks compared to workers developing in lower temperatures. Age can also be correlated with thermal-related risks, as it is known that ants with increasing age (and therefore decreasing life expectancy) perform riskier tasks (Schmid-Hempel and Schmid-Hempel 1984; Schmid-Hempel and Wolf 1988; Woyciechowski and Kozłowski 1998; Moroń et al. 2008, 2012; Miler et al. 2017). For this reason, we expected that with increasing age, workers may be more willing to forage in higher temperatures.
Materials and methods
Colonies in the experiment
Five colonies of F. cinerea were used in the experiments—from here on called original colonies. Three original colonies were collected in Romania (Cluj-Napoca) and two in Poland (Stara Wieś near the city of Warsaw) in the spring (May–June). Colonies were transported to the laboratory and housed in plastic boxes (20 × 30 × 40 cm). Each original colony had at least one fertile queen, offspring (eggs, larvae and pupae) in different proportions and several hundred workers (at least 300). Every original colony was checked once per week for last instar larvae (well developed, big larvae) that, if found, were removed to a new plastic box (20 × 30 × 10 cm), from here on called a temporal colony—created to easily observe the development of larvae into pupae. In each temporal colony, there were 20–80 last instar larvae along with 30 workers originating from their original colony. All original and temporal colonies were kept in thermal chambers with a controlled photoperiod (day-night: 14–10 h) and temperature (day-night: 23–16 °C). Water and food (crickets, Drosophila sp., honey with water) were provided ad libitum in an attached foraging arena (20 × 20 × 10 cm).
Temperature regime for pupal development
Temporal colonies were checked three times per week and the newly emerged pupae were separated and divided into two groups of an equal number and placed in two different thermal chambers. In natural conditions, pupae are usually kept in the warmest part of the nest (e.g. close to the surface) so workers can easily manipulate their development temperature by transporting them to different chambers in the nest (Weidenmüller et al. 2009; Falibene et al. 2016). Therefore, we also decided to use pupae—not larvae—in the experiment. One group of pupae was kept at 20 °C and the second at 28 °C during the day, while both chambers were kept at 15 °C at night (day-night: 14–10 h). The experimental regime of temperatures reflected ecologically relevant temperatures and were chosen based on our own experience and similar studies showing that the optimal development temperature for F. cinerea is about 29–30 °C (Weidenmüller et al. 2009; Seifert 2018). Each group of pupae was reared together with 5–10 workers that took care of the brood. These colonies were checked three times per week in order to capture the young acclimated workers emerging from the pupae. Young acclimated workers with an already hardened cuticle (usually 3–5 days after emerging) were individually paint-marked with two dots of various colours (Edding 751 marker). There was a total of 113 acclimated workers coming from the five colonies: colony FC1, 28 workers (12 from 20 °C and 16 from 28 °C); FC2, 21 (6 and 15, respectively), FC3, 9 (5 and 4 respectively), FC4, 37 (17 and 20, respectively), FC5, 18 (9 and 9, respectively). Altogether, there were 49 workers that developed in 20 °C and 64 from 28 °C. Subsequently, the acclimated workers that developed under different temperature regimes and originated from the same colony were merged together with a group of 40 unacclimated workers from the same original colony. None of the colonies contained a brood. Such experimental colonies were used to assess the temperature-related foraging risks of acclimated and unacclimated workers. However, at the end, after finishing the foraging risk tests, only four colonies (FC1, FC2, FC4, FC5) were used for the statistical analysis with the acclimated workers—colony FC3 was removed due to low worker activity. Data collected from all colonies were used for the statistical analysis performed for the unacclimated workers. The experimental colonies were kept in small boxes (15 × 20 × 10 cm) in thermal chambers with a controlled photoperiod (day-night: 14–10) and temperature (23 °C, day; 16 °C, night). Unfortunately, we were unable to measure acclimated workers to estimate their size, due to the high risk of damaging them while they were still alive and in most cases, it was impossible to do so after their death due to the random place and time of their demise inside the nest.
Testing temperature-related foraging risk
To assess the temperature-related foraging risk of workers, each experimental colony was connected by a silicone tube to a foraging arena (15 × 15 cm) with a layer of sand at its bottom. This sand layer was warmed by a heating lamp to the required temperature. A thermostat probe was hidden in the sand, measuring its temperature (with an accuracy of 1 °C) and this information was sent to the heating lamp (Trixie 100 W) placed above the arena. A Microsoft LifeCam Studio camera was also placed above the arena to record the tests in HD resolution (1280 × 720). During the tests, two ad libitum food baits were placed centrally in the heated arena, honey in one and chunks of one cricket in the other. After placing the food, we waited 15 min to give the ants time to start foraging and then the actual test started—regardless of the appearance of ants inside the foraging arena. During each 15-min test, the arena was heated to the required temperatures (30 °C, 35 °C, 40 °C, 45 °C, 50 °C) and two variables were measured: (1) the foraging activity of unacclimated workers was measured by totalling the number of unacclimated foragers in the arena—there were 15 snapshot (2–3 s) measurements per each temperature; (2) the foraging activity of acclimated workers was measured by individually tracking each painted ant and measuring their total foraging time in each temperature. Foraging time started when an ant entered the arena and ended when it returned to the tube connecting the foraging arena with the nest. Each experimental colony was tested once per week. A total of 10 repetitions per colony were made. During the first five repetitions, each foraging arena was heated to the following temperatures: 30 °C – 35 °C – 40 °C – 45 °C – 50 °C. Subsequently, during the last five repetitions, the order of the temperatures was changed (30 °C – 50 °C – 45 °C – 35 °C – 40 °C) to control for the possible decrease in the activity of ants at higher temperatures caused by the saturation of workers with food as the test progressed. The temperature of 30 °C was used as a control temperature based on our previous experiments on F. cinerea foraging strategies (Ślipiński 2017). To make ants more willing to forage, all experimental colonies were starved for 5 days prior to the experiments and they received food only during the experiments and on the day after. The foraging risk tests were conducted between 14.09.2018 and 29.11.2018.
The statistical approach
The effect of external temperature on the foraging activity (number) of unacclimated workers
As a first step, we investigated the influence of foraging temperature (arena temperature) on the averaged number (N = 182) of unacclimated foragers (Online Resource 1) using a linear mixed model approach (LMM, maximum likelihood fit). For the purpose of the statistical test, the number of unacclimated workers was averaged due to the large number of repetitions (N = 2726). In each temperature, there were 15 snapshot measurements; during each snapshot, the number of all ants occurring in the arena was totalled; finally, all snapshot measurements were totalled and divided by 15. The number of unacclimated foragers in the heated arena was used as a dependent variable; the foraging temperatures and the order of temperatures were used as fixed factors, whereas the colony and the repetition (10 per colony) were introduced as nested random factors.
The influence of pupal development temperature and worker age on the foraging activity (time) of acclimated workers
In the next step, we checked the influence of acclimated worker (Online Resource 2) age and foraging temperatures on foraging time (s) using the linear mixed model approach (LMM, maximum likelihood fit; N = 217). In the model, workers’ development temperature, age, foraging temperatures (arena temperature) and the interaction of the two latter were included as fixed factors, while colony and worker ID were the nested random factors.
To check the influence of the different development temperatures (20 °C, N = 68; 28 °C, N = 149) on the foraging time (s) of acclimated workers in the different foraging temperatures, we used two separate LMM models (maximum likelihood fit), one for ants that developed at 20 °C and the second model for ants that developed at 28 °C. In the models, the foraging temperature was a fixed factor; age was a covariate, while colony and worker ID were used as nested random factors.
We also compared the number of foraging episodes (observed values) of acclimated workers with different pupal development temperatures (20 °C and 28 °C) to the expected values, assuming no difference between these two groups of workers by using a χ2 goodness-of-fit test (and Yates’ continuity correction).
All statistical analyses were performed in the R Statistical Environment (R Development Core Team 2018). The models were performed using the lmer function from the lme4 package (Bates et al. 2015). The main effects of the variables were calculated using the Anova function from the car package (Fox and Weisberg 2019). The coefficients of determination for linear mixed-effect models were calculated using the r.squaredGLMM function from the MuMIn package (Bartoń 2019). Foraging time (s) was log-transformed to fit the normal distribution.
Results
The effect of external temperature on the foraging activity (number) of unacclimated workers
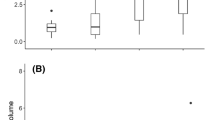
The number of unacclimated workers was significantly influenced by foraging (arena) temperature (χ2 = 78.92, p < 0.001) but not by the order of the applied temperatures (χ2 = 0.03, p < 0.852). Workers decreased their activity due to increasing temperature regardless of the order of the tests conducted during the experiments (possible effect of saturation with food). By analysing the different factor levels separately, we found that the foraging number of unacclimated workers decreased significantly with increasing temperature (35 to 50 °C, Table 1). The model explained 45% of the variance of the foraging number of unacclimated workers (Fig. 1).
The influence of pupal development temperature and worker age on the foraging activity of acclimated workers
The foraging time of acclimated workers was significantly influenced by foraging (arena) temperature (χ2 = 26.65, p < 0.001), worker’s age (χ2 = 15.18, p < 0.001), the interaction of these two predictors (χ2 = 13.87, p = 0.007), but not by worker’s development temperature (χ2 = 0.29, p = 0.6). When analysing the different factor levels separately, we found that the foraging time of acclimated workers was shorter in the riskiest temperatures of 45 °C and 50 °C (Table 2). With increasing age, workers foraged significantly longer at the highest (interaction between temperature and age), riskier temperatures (Table 2). Altogether, the model explained 35% of the variance of the foraging time of acclimated workers.
When analysing separately the changes in the foraging time of acclimated workers that developed at 20 °C, we found that the main effects of foraging temperature (χ2 = 5.43, p = 0.25) and age (χ2 = 0.09, p = 0.77) did not have a significant effect. However, these workers’ foraging time decreased significantly at 50 °C compared to the control temperature (Table 3). The model explained only 10% of the variance of the foraging time of acclimated workers that developed at 20 °C. On the other hand, the main effects of foraging temperature (χ2 = 20.54, p < 0.001) and age (χ2 = 15.38, p < 0.001) for workers that developed at 28 °C had highly significant effects. These workers foraged for significantly shorter times in all temperatures compared to the control temperature, but the time of foraging increased with their age (Table 3, Fig. 2). The model explained a total of 34% of the variance of the foraging time of acclimated workers that developed at 28 °C.
The effect of age (days) on the foraging time of acclimated workers that developed at 20 °C (left panel; t = 0.29, p = 0.77) and 28 °C (right panel; t = 3.92, p < 0.001). R2 values in the models analysing the effects of different values on the foraging time of workers developed at 20 °C (cond = 0.1, marg. = 0.07) and at 28 °C (cond = 0.34, marg. = 0.2). The blue lines with confidence bands (grey) are plotted based on the linear regression of the two variables
Pupal development temperature had a significant influence on the frequency of the foraging episodes of workers (Fig. 3), as those that developed at 28 °C foraged more often than ants that developed at 20 °C (χ2 = 16.85, p < 0.001).
Discussion
Our results demonstrated that a high surface temperature affects negatively the foraging activity of Formica cinerea workers and the majority of them do not forage when temperatures are high. However, there are behavioural differences among individuals that may be related to the temperature of their development and their age. Our results demonstrated that pupal development temperature influenced the foraging strategies of adult workers; workers that developed in the high temperature (28 °C) foraged more often but for shorter intervals compared to the workers that developed in the lower temperature (20 °C). We also found that the age of workers influences their foraging activity; with increasing age, workers are prone to forage significantly longer at the highest and riskiest temperatures.
Ants living in a temperate climate, such as F. cinerea, have different life-history traits compared to many typical desert ant species. Formica cinerea nests in sandy soil, e.g. in a forest clearing or dunes, and forage on sand (Czechowski et al. 2002; Ślipiński et al. 2015; Ślipiński 2017). It is not a scavenging species, like desert ants, although their workers forage on hot sand if they need to collect food, e.g. honeydew from plants growing at a forest edge. The temperature of the sand in such a forest clearing may reach 61 °C (Ślipiński 2017), a temperature that is above the thermal maximum of most foraging ants in temperate climates. On the other hand, foragers may stay inside the nest and wait until the sand surface cools down. Such a situation was observed in the field (Ślipiński 2017) and it is also supported by the results of our observations of the unacclimated F. cinerea workers, as their number decreased significantly with increasing surface temperatures. Generally, the activity of a F. cinerea colony decreases during the hottest part of the day, when the majority of workers stay inside the nest. However, during our experiment, we observed behavioural differences among workers in terms of foraging in high (risky) temperatures.
Organisms living in thermally heterogeneous environments can respond to temperatures during one stage of the life cycle to enhance performance during a subsequent stage (intra-generational response, Angilletta 2009). Such a response was presented in a handful of cases with the use of the larvae or pupae of honeybees or ant workers (Tautz et al. 2003; Jones et al. 2005; Weidenmüller et al. 2009; Falibene et al. 2016; Oms et al. 2017). Current knowledge about the influence of the proximate mechanisms that determine and maintain inter-individual behavioural variability in social insects, including risk-prone strategies, is still limited. Our study indicates that the development temperature of pupae may influence the foraging activity of adult workers; those that developed in a higher temperature foraged more often, which can indicate their higher temperature resistance, but they actually spent less time outside the nest compared to workers that developed in lower temperatures. Results from different experiments conducted on Aphenogaster senilis indicated that a higher brood development temperature is correlated with higher temperature resistance (Oms et al. 2017). In addition, a study on Drosophila melanogaster demonstrated that the development temperature of individuals influenced their heat tolerance; individuals that developed at a higher temperature (25 °C versus 18 °C) had a higher knock-down temperature (Crill et al. 1996). Differences in foraging behaviour between ant workers that developed in different temperature regimes could be beneficial for colony performance and it could be controlled by adult workers, which can partially determine the thermal conditions of brood development (see Oms et al. 2017). It was proven that controlling brood development temperature influences the morphological and behavioural traits of imago workers, e.g. brain development in Camponotus mus ants, which affects sensory processing and learning abilities in adult ants (Falibene et al. 2016). For F. cinerea, different foraging strategies and behavioural variation in the risk-prone strategies of workers that developed in various temperatures may be advantageous when competing with strong competitors occurring in the surrounding habitat (e.g. Formica wood ants).
The results of our study revealed that the age of workers can also be an important factor that is correlated with the foraging activity of workers in high temperatures. It is known that ant age is associated with task specialization and the division of labour within a colony (age polyethism). Young workers show a preference for within-nest tasks; thus, brood care falls mostly to them. Slightly older workers tend to do other tasks inside the nest, whereas the oldest ants perform the riskiest tasks of foraging and are the first to engage in colony defence, as ‘switching to more dangerous tasks is age-influenced’ (Hölldobler and Wilson 1990, 2009; Cerdá and Retana 1992; Sendova-Franks and Franks 1995; Tripet and Nonacs 2004; Tschinkel 2006; Lach et al. 2009). In addition, the results from our experiments indicate that workers risk more and forage longer with aging. Alternatively, it is also possible that ants with age become more heat tolerant, which implies that the level of risk may be similar for all age classes; just the threshold of overheating is higher in older ants. Interestingly, the factor of age is more significant for ants that developed in the higher temperature of 28 °C; these ants’ foraging time significantly increased with their age. The probability of death during foraging in a high temperature is much greater compared to foraging performed at lower temperatures or tasks performed inside the nest. Therefore, older workers, which usually also have a shorter life expectancy, should perform riskier tasks, as has been shown in many other studies on social insects (Schmid-Hempel and Schmid-Hempel 1984; Schmid-Hempel and Wolf 1988; Woyciechowski and Kozłowski 1998; Moroń et al. 2008, 2012; Miler et al. 2017).
There can also be other factors, not tested in this study, which can influence the behavioural variation of workers in their temperature-related risk prone strategies. F. cinerea has polymorphic individuals (Czechowski et al. 2002), and therefore, worker size could potentially be an important factor. It was shown that larger workers are supposed to be more thermally resistant due to their lower surface-to-volume ratio, lower mass-specific metabolic rate and longer legs (Shik 2010; Cerdá and Retana, 1997, 2000; Villalta et al. 2020).
It is predicted that insects from temperate regions, compared to tropical ones, may be less exposed to climate change risk (Deutsch et al. 2008; Andrew et al. 2013; Sunday et al. 2014; Diamond and Chick 2018; Johnson et al. 2020), but this assumption has been poorly examined. For a better understanding of such a process, it would be necessary to conduct further studies taking into account the microclimatic variation and the behavioural optimisation of animals (e.g. Anderson et al. 1979; Suggitt et al. 2011; Andrew et al. 2013). Moreover, our knowledge about the level of the behavioural and physiological adaptations of species from temperate climates for coping with high surface temperatures is limited, compared to the broad knowledge on typical thermal specialists from warmer regions (Christian and Morton 1992; Cerdá 2001; Schultheiss and Nooten 2013; Willot et al. 2017). There is also limited data on the influence of climate change on the foraging strategies and physiological adaptations of ants from temperate ecosystems that forage at their upper thermal limits, although some information is provided by studies performed in Asia. It was demonstrated that Formica exsecta, which was a relatively rare and sporadically distributed species in the region of the Kolyma River (Asian part of Russia), became the dominant species of the mesofauna of this region, which is the effect of the warm winters in the last decade (Alfimov et al. 2011). The influence of climate change was also reported in the taiga during unusually hot periods in the summer of 2010. Studied colonies of the genus Formica responded to the extreme heat by changing their foraging patterns, redesigning their nests and rearranging the spatial and functional structure of the colonies. They also switched to a bimodal daily activity pattern with the maxima in the morning and in the evening and a prolonged daytime intermission (Zakharov and Zakharov 2014). Nevertheless, more studies should be conducted to be able to predict how ants and other insects from temperate climates can adapt their foraging behaviour to current climate change and to identify species and populations endangered by decline or extinction.
Data availability
The data generated during the study are available as supplementary material.
References
Alfimov AV, Berman DI, Zhigulskaya ZA (2011) Fluctuation in the abundance of the narrow-headed ant (Formica exsecta, Hymenoptera, Formicidae) and climatic changes in the northeastern part of its range. Entomol Rev 91:177–188. https://doi.org/10.1016/j.gloplacha.2020.103149
Anderson RV, Tracy CR, Abramsky Z (1979) Habitat selection in two species of short-horned grasshoppers. Oecologia 38:359–374. https://doi.org/10.1007/BF00345194
Andrew NR, Hart RA, Jung M-P, Hemmings Z, Terblanche JS (2013) Can temperate insects take the heat? A case study of the physiological and behavioural responses in a common ant, Iridomyrmex purpureus (Formicidae), with potential climate change. J Insect Physiol 59:870–880. https://doi.org/10.1016/j.jinsphys.2013.06.003
Angilletta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, Oxford
Bartoń K (2019) MuMIn: Multi-model inference. R package version 1.43.6. https://CRAN.R-project.org/package=MuMIn
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F (2012) Impacts of climate change on the future of biodiversity. Ecol Lett 15:365–377. https://doi.org/10.1111/j.1461-0248.2011.01736.x
Bernstein RA (1974) Seasonal food abundance and foraging activity in some desert ants. Am Nat 108:490–498
Bernstein RA (1979) Schedules of foraging activity in species of ants. J Anim Ecol 48:921–930
Boulay R, Aron S, Cerdá X, Doums C, Graham P, Hefetz A, Monnin T (2017) Social life in arid environments: the case study of Cataglyphis ants. Annu Rev Entomol 62:305–321. https://doi.org/10.1146/annurev-ento-031616-034941
Cerdá X (2001) Behavioural and physiological traits to thermal stress tolerance in two Spanish desert ants. Etología 9:15–27
Cerdá X, Retana J (1992) A behavioural study of transporter workers in Cataglyphis iberica ant colonies (Hymenoptera Formicidae). Ethol Ecol Evol 4:359–374. https://doi.org/10.1080/08927014.1992.9523128
Cerdá X, Retana J (1997) Links between worker polymorphism and thermal biology in a thermophilic ant species. Oikos 78:467–474
Cerdá X, Retana J (2000) Alternative strategies by thermophilic ants to cope with extreme heat: individual versus colony level traits. Oikos 89:155–163. https://doi.org/10.1034/j.1600-0706.2000.890117.x
Cerdá X, Retana J, Cros S (1998) Critical thermal limits in Mediterranean ant species: trade-off between mortality risk and foraging performance. Funct Ecol 12:45–55. https://doi.org/10.1046/j.1365-2435.1998.00160.x
Chung YK, Lin CC (2017) Heat-induced symmetry breaking in ant (Hymenoptera: Formicidae) escape behavior. PLoS ONE 12(3):e0173642. https://doi.org/10.1371/journal.pone.0173642
Clémencet J, Cournault L, Odent A, Doums C (2010) Worker thermal tolerance in the thermophilic ant Cataglyphis cursor (Hymenoptera, Formicidae). Insect Soc 57:11–15. https://doi.org/10.1007/s00040-009-0044-y
Crill WD, Huey RB, Gilchrist GW (1996) Within- and between-generation effects of temperature on the morphology and physiology of Drosophila melanogaster. Evolution 50:1205–1218. https://doi.org/10.1111/j.1558-5646.1996.tb02361.x
Christian KA, Morton SR (1992) Extreme thermophilia in a Central Australian ant, Melophorus bagoti. Physiol Zool 65:885–905
Czechowski W, Radchenko A, Czechowska W (2002) The ants of Poland. Museum and Institute of Zoology PAS, Warsaw
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC et al (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA 105:6668–6672. https://doi.org/10.1073/pnas.0709472105
Diamond SE, Chick L (2018) The Janus of macrophysiology: stronger effects of evolutionary history, but weaker effects of climate on upper thermal limits are reversed for lower thermal limits in ants. Current Zool 64:223–230. https://doi.org/10.1093/cz/zox072
Evgen’ev MB, Garbuz DG, Shilova VY, Zatsepina OG (2007) Molecular mechanisms underlying thermal adaptation of xeric animals. J Biosc 32:489–499. https://doi.org/10.1007/s12038-007-0048-6
Falibene A, Roces F, Rössler W, Groh C (2016) Daily Thermal fluctuations experienced by pupae via rhythmic nursing behavior increase numbers of mushroom body microglomeruli in the adult ant brain. Front Behav Neurosci 10:1662–5153. https://doi.org/10.3389/fnbeh.2016.00073
Fox J, Weisberg S (2019) An {R} Companion to Applied Regression, Third Edition. Sage, Thousand Oaks. URL: https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Friedman DA, Greene MJ, Gordon DM (2019) The physiology of forager hydration and variation among harvester ant (Pogonomyrmex barbatus) colonies in collective foraging behavior. Sci Rep 9:5126. https://doi.org/10.1038/s41598-019-41586-3
Gehring WJ, Wehner R (1995) Heat-shock protein-synthesis and thermotolerance in Cataglyphis, an ant from the Sahara desert. Proc Natl AcadSci USA 92:2994–2998. https://doi.org/10.1073/pnas.92.7.2994
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Hölldobler B, Wilson EO (2009) The superorganism. W.W. Norton, New York
IPCC (2018) Summary for policymakers. In Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre‐industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. World Meteorological Organization, Geneva
Jeanson R, Weidenmüller A (2014) Interindividual variability in social insects-proximate causes and ultimate consequences. Biol Rev 89:671–687. https://doi.org/10.1111/brv.12074
Johnson NC, Amaya DJ, Ding Q, Kosaka Y, Tokinaga H, Xie SP (2020) Multidecadal modulations of key metrics of global climate change. Glob Planet Chang 188:103149. https://doi.org/10.1016/j.gloplacha.2020.103149
Jones JC, Helliwell P, Beekman M, Maleszka R, Oldroyd BP (2005) The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191:1121–1129. https://doi.org/10.1007/s00359-005-0035-z
Lach L, Parr C, Abbott K (2009) Ant ecology. Oxford University Press, New York
Merkt JR, Taylor CR (1994) “Metabolic switch” for desert survival. Proc Natl Acad Sci USA 91:12313–12316. https://doi.org/10.1073/pnas.91.25.12313
Miler K, Symonowicz B, Godzińska EJ (2017) Increased risk proneness or social withdrawal? The effects of shortened life expectancy on the expression of rescue behavior in workers of the ant Formica cinerea (hymenoptera: formicidae). J Insect Behav 30:632–644. https://doi.org/10.1007/s10905-017-9647-8
Moroń D, Witek M, Woyciechowski M (2008) Division of labour among workers with different life expectancy in the ant Myrmica scabrinodis. Anim Behav 75:345–350. https://doi.org/10.1016/j.anbehav.2007.06.005
Moroń D, Lenda M, Skórka P, Woyciechowski M (2012) Short-lived ants take greater risks during food collection. Am Nat 180:744–750. https://doi.org/10.1086/668009
Oms CS, Cerdá X, Boulay R (2017) Is phenotypic plasticity a key mechanism for responding to thermal stress in ants? Naturwissenschaften 104(5–6):42. https://doi.org/10.1007/s00114-017-1464-6
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42. https://doi.org/10.1038/nature01286
Perez R, Aron S (2020) Adaptations to thermal stress in social insects: recent advances and future directions. Biol Rev 95:1535–1553. https://doi.org/10.1111/brv.12628
Piantoni C, Navas CA, Ibargüengoytía NR (2019) A real tale of Godzilla: impact of climate warming on the growth of a lizard. Biol J Linn Soc 126:768–782. https://doi.org/10.1093/biolinnean/bly216
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org
Schultheiss P, Nooten SS (2013) Foraging patterns and strategies in an Australian desert ant. Austral Ecol 38:942–995. https://doi.org/10.1111/aec.12037
Schmid-Hempel P, Schmid-Hempel R (1984) Life duration and turnover of foragers in the ant Cataglyphis bicolor (Hymenoptera: Formicidae). Insect Soc 31:345–360
Schmid-Hempel P, Wolf T (1988) Foraging effort and life span of workers in a social insect. J Anim Ecol 57:509–521
Seifert B (2018) The ants of central and north Europe. – lutra Verlags – und Vertriebsgesellschaft, Tauer, Germany
Sendova-Franks AB, Franks NR (1995) Spatial relationships within nests of the ant Leptothorax unifasciatus (Latr.) and their implications for the division of labour. Anim Behav 50:121–136. https://doi.org/10.1006/anbe.1995.0226
Shik JZ (2010) The metabolic costs of building ant colonies from variably sized subunits. Behav Ecol Sociobiol 64:1981–1990. https://doi.org/10.1007/s00265-010-1009-x
Suggitt AJ, Gillingham PK, Hill JK, Huntley B, Kunin WE, Roy DB, Thomas CD (2011) Habitat microclimates drive fine-scale variation in extreme temperatures. Oikos 120:1–8. https://doi.org/10.1111/j.1600-0706.2010.18270.x
Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB (2014) Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc Natl Acad Sci USA 111:5610–5615. https://doi.org/10.1073/pnas.1316145111
Ślipiński P (2017) Temperature-related foraging risk in temperate climate ants (Hymenoptera: Formicidae). North-Western J Zool 13:1–5
Ślipiński P, Pomorski JJ, Kowalewska K (2015) Heat shock proteins expression during thermal risk exposure in the temperate xerothermic ant Formica cinerea. Sociobiology 62:457–459. https://doi.org/10.13102/sociobiology.v62i3.409
Tautz J, Maier S, Groh C, Rössler W, Brockmann A (2003) Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc Natl Acad Sci USA 100:7343–7347. https://doi.org/10.1073/pnas.1232346100
Thackeray SJ, Henrys PA, Hemming D, Bell JR, Botham MS, Burthe S, Wanless S (2016) Phenological sensitivity to climate across taxa and trophic levels. Nature 535:241–245. https://doi.org/10.1038/nature18608
Tripet F, Nonacs P (2004) Foraging for work and age based polyethism: the roles of age and previous experience on task choice in ants. Ethology 110:863–877. https://doi.org/10.1111/j.1439-0310.2004.01023.x
Tschinkel WR (2006) The fire ants. The Belknap Press of Harvard University Press, Cambridge
Villalta I, Oms CS, Angulo E, Molinas-González CR, Devers S, Cerdá X, Boulay R (2020) Does social thermal regulation constrain individual thermal tolerance in an ant species? J Anim Ecol 89:2063–2076. https://doi.org/10.1111/1365-2656.13268
Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395. https://doi.org/10.1038/416389a
Wehner R, Marsh AC, Wehner S (1992) Desert ants on a thermal tightrope. Nature 357:586–587. https://doi.org/10.1038/357586a0
Weidenmüller A, Mayr C, Kleineidam CJ, Roces F (2009) Preimaginal and adult experience modulates the thermal response behavior of ants. Curr Biol 19:1897–1902. https://doi.org/10.1016/j.cub.2009.08.059
Wersebe M, Blackwood P, Guo YT et al (2019) The effects of different cold temperature regimes on development, growth, and susceptibility to an abiotic and biotic stressor. Ecol Evol 9:3355–3366. https://doi.org/10.1002/ece3.4957
Willot Q, Gueydan C, Aron S (2017) Proteome stability, heat hardening and heat-shock protein expression profiles in Cataglyphis desert ants. J Exp Biol 220:1721–1728. https://doi.org/10.1242/jeb.154161
Woyciechowski M, Kozłowski J (1998) Division of labor by division of risk according to worker life expectancy in the honey bee (Apismellifera L.). Apidologie 29:191–205. https://doi.org/10.1051/apido:19980111
Zakharov AA, Zakharov RA (2014) Ants under the conditions of an extremely hot summer. Entomol Rev 94:526–540. https://doi.org/10.1134/S0013873814040071
Funding
The study was supported by the Polish National Science Centre, grant no. 2015/17/B/NZ8/02492.
Author information
Authors and Affiliations
Contributions
PŚ and MW designed the experiment. All authors conducted the experiment. PŚ and IM analysed the data. PŚ wrote the draft of the manuscript, which was later corrected by all authors.
Corresponding author
Ethics declarations
Ethics approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by W. Hughes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ślipiński, P., Trigos-Peral, G., Maák, I. et al. The influence of age and development temperature on the temperature-related foraging risk of Formica cinerea ants. Behav Ecol Sociobiol 75, 107 (2021). https://doi.org/10.1007/s00265-021-03029-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-021-03029-w