Abstract
Understanding intraspecific phenotypic variation in prey specialisation can help to predict how long-term changes in prey availability affect the viability of these phenotypes and their persistence. Generalists are favoured when the main food resources are unpredictable compared to specialists, which track the availability of the main prey and are more vulnerable to changes in the main food resource. Intraspecific heritable melanin-based colour polymorphism is considered to reflect adaptations to different environments. We studied colour morph-specific diet specialisation in a generalist predator, tawny owl (Strix aluco), during offspring food provisioning in relation to mammal prey density. We hypothesised that the grey morph, with higher fitness than the brown in Northern boreal conditions, is more specialised in mammalian prey than the brown morph, which in turn has higher fitness than the grey in the temperate zone. We found a higher diversity of prey delivered to the nest by brown fathers compared to grey ones, which also depended on the overall mammalian prey availability. Brown fathers provided proportionally fewer mammalian prey than grey in poor, but not in favourable mammal prey years. Our results suggest that the brown morph is more generalistic and reacts more strongly to variations in food supply than the grey morph, which may be a beneficial strategy in an unpredictable environment caused by environmental degradation.
Significance statement
Diet choice of a species may vary depending on fluctuations in the abundance of their food resource, but also within a population, there can be adaptations to use different food resources. The tawny owl exhibits a grey and a reddish-brown colour morph and is considered a generalist predator eating both mammal and bird prey. We find that the diet of the reddish-brown morph is more diverse than that of the grey. When the tawny owls’ main prey, small mammals, are abundant both colour morphs prey on mammals, but in years with less small mammals, the reddish-brown morph is more prone of switching to small bird predation than the grey. The generalist strategy of the brown morph is likely to be more favourable than a stricter specialisation in small mammals of the grey under recently reoccurring irregularities in small mammal dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diet specialisation and phenotypic variation in food resource use and food requirements dictate the ability of populations and species to respond to changes in food resource availability. Theoretically, the degree of specialisation can range from pure “narrow-range resource utilising specialists” to “wide-range resource utilising generalists”, and this continuum can occur both within- and between-species (Partridge and Green 1985; Wilson and Yoshimura 1994; Durell 2000; Bernays et al. 2004; Egan and Funk 2006; Woo et al. 2008).
In predatory species, optimal foraging models predict that diet specialisation is determined by the variety and abundance of available prey and by the energetic value of utilising the prey, e.g. search time in relation to abundance, handling time and caloric value (MacArthur and Pianka 1966). It is therefore predicted that the degree of specialisation is a function of spatial and temporal heterogeneity and predictability in the abundance of resources, cultural experiences and the efficiency of foraging adaptations (Partridge and Green 1985; Sherry 1990; Whitfield 1990; Durell 2000). Specialists, which evolve specific adaptations or breeding strategies, do better when their preferred food resource is abundant, but may face a cost of specialisation if the preferred prey becomes scarce (Terraube et al. 2011). Therefore, generalists are predicted to be favoured when the main food resources are unpredictable or scarce, whereas specialists adjust their life history to the availability of the main prey and become more vulnerable to unpredictable changes in the main food resource.
How diet specialisation varies within species and how it evolves and is maintained remain poorly understood. Such knowledge of heritable phenotypic and/or genetic diversity in resource use in natural populations would help to understand how directed changes in food resources due to environmental change could impact population dynamics, diversity and viability. Empirical studies of species displaying genetic polymorphisms can provide novel insight of heritable intraspecific diversity in food resource use and specialisation and thereby improve our knowledge of the evolutionary potential and ecological relevance of intraspecific diet specialisation.
The general theory of genetic polymorphisms states that polymorphism evolves and can be maintained in environments, which vary in time or space and where the fitness of the alternative morphs depends on the environment (Ford 1945; Fisher 1958; Majerus 1998). Selection therefore favours differential use of the habitat or niche by the morphs, which is expected to be reflected in e.g. their life history and choice of diet (Galeotti and Rubolini 2003; Roulin 2004). A phenotypic marker for genetic polymorphism in vertebrates—melanin-based colour polymorphism—has been found to covary with several behavioural and physiological traits (Ducrest et al. 2008) with fitness depending on the environment they are experiencing (Svensson et al. 2001; Karell et al. 2011a; Emaresi et al. 2014; San-José et al. 2019). This makes colour polymorphic species excellent study organisms to investigate heritable intraspecific diversity in resource use specialisation.
In this paper, we study prey specialisation in the colour polymorphic tawny owl (Strix aluco) in a natural population in Finland in 2013–2018. In the boreal zone, tawny owls mainly prey on voles, which fluctuate in numbers between years (Karell et al. 2009a) and which have been found to follow irregular dynamics in recent years (Hörnfeldt et al. 2005; Brommer et al. 2010; Cornulier et al. 2013). In years with poor availability of small mammals, tawny owls switch to utilise more alternative prey, mainly birds (Petty 1999), and they produce smaller broods or may skip breeding completely (Roulin et al. 2003; Karell et al. 2009a; Millon et al. 2014). The tawny owls come in a reddish-brown (hereafter brown) and a grey highly heritable morph (Brommer et al. 2005; Karell et al. 2011a), which reside in the same geographical area and mate non-assortatively with respect to plumage colour (Brommer et al. 2005). Both colour morphs produce a similar number of offspring per reproductive event in northern boreal environments, but the grey morph has higher fitness than the brown morph due to higher survival (Brommer et al. 2005) especially in years of snow-rich and cold winter conditions (Karell et al. 2011a). On the contrary, the brown morph has higher fitness in a central European population under experimentally improved offspring rearing conditions (Emaresi et al. 2014). In Finland, the brown morph has steadily increased in frequency since the 1960s in tandem with milder winter conditions (Karell et al. 2011a).
Tawny owls have biparental care with distinct division of duties: The smaller males engage in courtship feeding of the female prior to egg-laying and are the main providers of food for the incubating female and hatched offspring, whereas the larger females incubate the eggs and guard the nest (Mikkola 1983; Sasvári et al. 2000; Sunde et al. 2003). During breeding, the brown tawny owls have a more fixed reproductive effort than the grey (Emaresi et al. 2014), and the brown morph parents produce heavier offspring regardless of fluctuations in abundance of small mammal prey (Morosinotto et al. 2020). In central Europe, grey tawny owl females are more likely than brown to skip breeding in unfavourable years (Roulin et al. 2003), and grey offspring grow relatively better in adverse food conditions (Piault et al. 2009). These results suggest that reproductive effort of the colour morphs is differently determined and therefore that they would be differently affected by changes in food resource availability. Brown tawny owls may be better than the grey at utilising the microhabitat, either in terms of space use or daily activity rhythm, which is likely to translate into differential prey choice (Galeotti and Rubolini 2003).
Here, we test the hypothesis that the brown morph has a wider diet breadth than the grey morph and that the grey morph is more specialised on specific types of prey. In the boreal zone, microtine voles fluctuate strongly in cycles, and many birds of prey are dependent on peaks in vole numbers for reproduction (Brommer et al. 2000, 2002; Karell et al. 2009a; Lehikoinen et al. 2011; Korpimäki and Hakkarinen 2012). Furthermore, during winter, most bird prey species migrate south, which makes overwinter survival highly dependent on mammal prey (Francis and Saurola 2004), and we therefore predict that specialisation on mammal prey would be favoured by selection in this northern environment. We hypothesise that the grey tawny owl morph is more specialised on mammalian prey, whereas the brown morph has a wider prey use, switching more easily to alternative prey, and is thereby less dependent on a specific food resource.
Material and methods
The study was conducted in Uusimaa, at a study area with suitable tawny owl nest boxes of approximately 300 km2 (centre of study area 60.24696, 24.28244). The area consists of a mosaic of agricultural land, spruce-dominated and mixed forests and lakes and streams. The landscape and habitat configuration is similar in territories inhabited by grey and brown tawny owl males (Schenk 2018). Approximately one hundred nest boxes were placed in mixed forest areas with a relatively high proportion of deciduous trees. Nest sites were visited 3–5 times during the breeding season to collect information about clutch size, timing of hatching and hatching success and to conduct parental trapping and offspring ringing prior to fledging (see Karell et al. (2009a) for details on general protocol). Brood size equals the number of ringed fledglings. Both parents were trapped at the nest box during the nestling period, aged, measured (wing length and mass) and ringed to allow individual identification (Karell et al. 2009a). Plumage colour was scored in adults using a semi-continuous score based on the degree of reddish-brown colouration in four body parts: facial disc, back, breast and general appearance. From this overall score, we then categorised each individual as either grey or brown morph (Brommer et al. 2005). It was not possible to record morphometric data blind because our study involved focal animals in the field.
After each breeding event, the nest material (sawdust-wood chips layer in which the prey remains and regurgitated pellets accumulate) is collected and replaced with a fresh layer in each nest. Tawny owl young regurgitate the undigested parts of the prey, bones and fur or feathers in pellets, which accumulate and mix in with the nest material. In 2013–2018, we identified the prey remains from 38 nests in which both parents were identified, and which successfully produced fledglings.
The sawdust and prey remains were sorted according to the method described in Brommer et al. (2003) and Kekkonen et al. (2008). In short, bones were defined as right and left side when possible and paired, which yields a least estimation of individual numbers. Mammals were identified on species or genus level, while birds were sorted into groups according to their size: large birds, thrush-sized birds, finch-sized birds and smaller warbler-sized birds following the guidelines by Juutilainen (1998), Siivonen and Sulkava (2002) and Bjärvall and Ullström (2010). The number of individuals of each species or group was counted from the most numerous bones. To minimise observer bias, blinded methods were used when prey remains were recorded and analysed.
In order to get an estimate of the prey mass of the different prey types delivered to the nest, we multiplied average mass of species or group (Supplementary Table 1) with the number of items counted from the remains (average number of prey delivered: 56.9 ± 5.7 SD). The number of items is always the least estimation, as the mother, which shares the food in the nest box, may eat some of the prey and regurgitate the pellets outside of the nest box. In addition, the smallest bone parts may be lost. This method gives good estimates of the parental prey delivery in Strix owls (Brommer et al. 2003; Karell 2007; Kekkonen et al. 2008; Karell et al. 2009b).
We studied the mass of the prey delivered per nest instead of number of prey items as this better corresponds to dietary requirements, resource use and net energetic value of the prey. For example, mammalian prey of tawny owls in our study area range commonly between 7 g (common shrew, Sorex araneus) and 257 g (rat, Rattus norvegicus) and everything in between (see Supplementary Table 1). For this reason, prey mass gives a more complete estimate of the diet the tawny owls provide to their nestlings compared to the number of prey items.
In tawny owls, males are the main food providers for the brood, whereas the females incubate and defend the nest (Mikkola 1983; Sasvári et al. 2000; Sunde et al. 2003). Males engage in courtship feeding of the female to attract her to lay eggs (Mikkola 1983). In this way males have a significant impact on the timing of breeding in tawny owls (Brommer et al. 2015). Halfway in the nestling period, when the chicks begin to thermoregulate well and are able to eat the prey items independently, the female begins to guard the nest from the outside and also contribute in food provisioning. Here, we consider that the males contribute to the reproductive output of the female (brood size) and that variation in prey delivered to the nest in the nestling period is mainly attributed to characteristics in the males (see “Statistical methods” section below).
Small mammal density was estimated each year in late autumn prior to snow fall by snap trapping in two sites within the study area, each containing both a field and a forest habitat. The small mammal density reflects reproductive decisions in the tawny owl in this population (Karell et al. 2009a). These two localities are expected to reflect the general seasonal abundance of small mammals in the study area since the spatial variation captured with this trapping method is far smaller than the annual variation (Karell et al. 2009b). The small mammals consisted mainly of bank voles (Myodes glareolus), field voles (Microtus agrestis), wood mice (Apodemus flavicollis) and shrews (Sorex spp.). Small mammal index in late autumn prior to the breeding season predicts reproductive output and diet of Strix owls (Brommer et al. 2002; Karell 2007; Kekkonen et al. 2008; Karell et al. 2009a, b). Small mammal prey index was calculated as the number of captures per 100 trap nights (Karell et al. 2009a). We did not have the possibility to assess the density of bird prey.
Statistical methods
Based on the prey items found, we calculated the Simpson’s diversity index (SDI) which takes into account the number, abundance and evenness of prey types (Simpson 1949). We present the inverse SDI (1/SDI) where a higher value indicates a higher diversity of prey types. We identified 13 mammalian prey types, 4 avian prey types, one lizard prey type and one frog prey type. For a full list of prey types found in the nests and used to calculate the inverse SDI, see Supplementary Table 1. For the second set of models, we separated the mass of birds and mammals respectively from the total prey mass to get estimates of proportion of bird prey mass and mammal prey mass. The inverse SDI does not reflect the type of prey (avian or mammalian), and therefore we analysed the proportions of the mass of different prey types (birds, mammals) in a different set of models.
We used linear mixed models with normal errors to analyse variation in inverse SDI and generalised linear mixed models with binomial errors to analyse the proportions of mammalian prey mass delivered to the nest. Brood size was entered as a covariate to correct for variation in reproductive effort. To account for repeated observations, we added Father ID since some fathers nested in several years (N = 28 fathers on 38 observations) and year as random effects (N = 6 years). All models (inverse SDI and proportion of avian/mammalian prey) included the same explanatory variables to aid in comparison of effects: father morph (grey = 1, brown = 2), small mammal index + brood size and the interaction father morph by small mammal index. In order to confirm that the effect was driven by the father morph and less by the mother morph, we also run an additional set of the same models where we replaced father morph with a four-level categorical variable “parental colour morph cross” (grey × grey, grey × brown, brown × grey, brown × brown; see also Morosinotto et al. 2020). This way we could assess the effect of both mother and father cross in the same variable (see supplement for results on these models). All models were implemented in R3.6.1 using the lmer function for the LMMs and glmer for the GLMMs in the lme4 package (R Development Core Team 2019). F-tests were used in significance testing of the LMMs and z-tests in the GLMMs. All models were checked for normality.
Results
On average, the tawny owls in our population delivered 3255 g (± 295 SE) of prey to their nest during the nestling period. Mammals constituted the largest mass in the delivered prey with 56% of total prey mass, 39% of the mass were birds, and 5% constituted of frogs and one lizard. In terms of prey types, the water voles (Arvicola amphibius), field voles (Microtus agrestis), bank voles (Myodes glareolus) and wood mice (Apodemus flavicollis) were the most important mammalian prey constituting 42.4% of the total prey mass. Among birds, thrushes were the biggest group with a total of 33.4% of the total prey mass (Supplementary Table 1).
Prey mass delivered to the nest showed no difference between father morphs (colour morph: F = 1.84, df = 30.36, p = 0.19), nor depending on the mammal index (small mammal index: F = 3.37, df = 3.12, p = 0.15) or their interaction (colour morph by small mammal index: F = -0.62, df = 32.0, p = 0.44). However, brood size was positively associated with prey mass (brood size: F = 18.23, df = 24.82, p < 0.001).
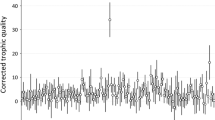
The diversity (number and evenness) of prey item types, estimated as the inverse Simpson’s diversity index (inverse SDI), was higher in nests sired by brown males than in those of grey males (Table 1, colour morph). Prey type diversity tended to be higher in years with higher small mammal abundance (Table 1, small mammal index), and this trend was strong only in nests sired by grey and less in those sired by brown males (Fig. 1; Table 1, colour morph by mammal index). Prey type diversity was not related to brood size (Table 1, brood size). The inverse SDI was not related to the morph of the mother (Supplementary Table 3).
The inverse Simpson’s diversity index of the prey items delivered by grey and brown fathers in relation to small mammal density index. A high value indicates high diversity (number and evenness) of prey types. The plot presents the marginal effects (± 95% CI) of the interaction in the statistical model (see “Material and Methods” section for details)
Grey fathers delivered a larger proportion of mammalian prey mass to their nestlings compared to brown fathers (Table 2a, colour morph). This effect depended on small mammal density, where the proportion of mammals in the diet delivered by brown males was lowest in low small mammal density and reached the level of the grey males in high small mammal densities (Fig. 2; Table 2a, colour morph by small mammal index). The proportion of mammal prey was higher in larger broods (Table 2a, brood size). The morph of the mother did not affect the proportion of mammalian prey mass (Supplementary Table 4).
Discussion
Within-species phenotypic variation in diet specialisation and its impact on population viability and adaptation is poorly understood. In order to achieve such information, we need empirical studies of natural populations where this variation can be monitored. Evidence is accumulating that colour polymorphism—a phenotypic marker for genetic polymorphism—is associated with morph-specificity in habitat use (Galeotti and Cesaris 1996; Dreiss et al. 2011; Passarotto et al. 2018; Koskenpato et al. 2020) and differential fitness depending on environmental conditions (Karell et al. 2011a; Emaresi et al. 2014; San-José et al. 2019). Such differences are expected to reflect variations in food resource use and diet specialisation. Here, we tested the hypothesis that colour polymorphism is linked with diet specialisation. We found that the diversity of prey delivered by tawny owl males to their nestlings differed depending on their colour morph and prevailing density of their staple food, small mammals. Prey brought by brown fathers had a higher diversity compared to that of the grey fathers, and this prey diversity variation between morphs depended on the overall mammalian prey availability.
Furthermore, we found that the type of prey delivered differed depending on the colour morph of the father: Brown fathers provided proportionally less mammalian prey compared to the grey in poor vole years but not in favourable small mammal years. Conversely, in poor vole years, the brown morph preyed mainly on the other main prey type, birds. Our results therefore suggest that the grey morph is more specialised in small mammal prey, whereas the brown morph is more generalistic and also switches to alternative prey to a larger extent than the grey morph when the mammalian prey densities are low. The morph of the mother did not have a significant impact on prey composition (Supplementary Tables 3 and 4), which is expected since the male is the main provider of food during the nestling stage (Sunde et al. 2003). Likewise, a limitation of the data collection method is that smaller prey items are more difficult to detect and some pellets may be removed by the parents, which may bias the observations to larger prey. However, both bird and mammal prey types that were identified in the data are large and easily detectable from their bones. Therefore, we are confident that our data reflect prey choice of tawny owl males.
The alternative foraging strategy hypothesis to explain the evolution and maintenance of colour polymorphism states that alternative colour morphs exploit different ecological niches (Roulin 2004). According to the pleiotropy hypothesis, regulation of melanin production in the melanocortin system in vertebrates simultaneously affects functions associated with behaviour and physiology (Ducrest et al. 2008; Emaresi et al. 2013). A plethora of empirical studies have found evidence suggesting covariation in wild populations regarding colour morph-specific physiology and immune function (Gasparini et al. 2009; Piault et al. 2009; Karell et al. 2011b, 2017; Almasi et al. 2012; Emaresi et al. 2013), dispersal, aggressiveness, habitat use (Galeotti and Cesaris 1996; Dreiss et al. 2011; van den Brink et al. 2012; Da Silva et al. 2013) and plumage characteristics (Roulin et al. 2013; Koskenpato et al. 2016). These intrinsic attributes are expected to directly affect behaviour and energy requirements, which translate into context-dependent diet preferences and adjustments of life history trade-offs of the colour morphs. Our results here are in line with this prediction and bring about novel results linking melanin-based colouration with diet specialisation. In particular, our results suggest that higher degree of pheomelanism (i.e. brown morph) is associated with a wider diet breadth.
The observed difference between morphs in diet breadth may be determined by a different use of the microhabitat between the two morphs. The “niche divergence hypothesis” (Galeotti and Rubolini 2003) states that morphs differently use the microhabitat which then in turn reflects different prey choices. Furthermore, differential activity rhythms of both the owls and their prey may affect the observed predation dynamics. Several species of small mammal prey (voles and shrews) are mainly active during night, while the bird species commonly found (various species of thrushes) are mainly diurnal. Our results clearly show that the brown morph utilises the second staple food, bird prey, to a larger extent in poor mammal years as compared to the grey morph. Small mammal prey are mainly found on the ground, whereas bird prey are mainly perching or flying. It is therefore possible that the brown morph has a different hunting strategy and utilises a wider set of microhabitats for hunting and/or has a wider activity rhythm and hunt more during daytime than grey ones. In such a scenario, the colour morphs would not necessarily be directly specialised in given diets but in the use of different ecological niches. If the spatial heterogeneity is similar, the two morphs would have a similar trophic niche as there would not be any specific advantage for any of them. But, in poor small mammal years, when also other environmental characteristics are likely to be different, the brown morph might have an advantage because it is able to exploit a wider range of environmental conditions, whereas the grey one is constrained by its ”specificity” to certain conditions and thereby utilise a narrower trophic niche. On the other hand, in harsh winters when bird prey has migrated south, the grey morph would have an advantage as a mammal specialist to increase its over-winter survival probability. Future studies should therefore aim at investigating prey choice and use of the habitat by the morphs outside the breeding season.
Differences in degree of specialisation are considered to be related to neural limitations in relation to diet breadth. For example, in bees specialist-species are more sensitive to key stimuli, whereas generalists respond equally to large quantities of sensory neural inputs (Dukas and Real 1991). According to the neural hypothesis of diet breadth (Bernays 1998), selecting an appropriate behaviour is more efficient if simple or exaggerated cues can be used as a basis of decision-making rather than making a choice among many complex sensory inputs. Experiments on grasshoppers show that there is a significant time cost of making a choice, relative to situations in which individuals have no choice (Bernays 1998). Therefore, choosing between different prey types increases decision times, which may involve a significant ecological risk. Quick decisions therefore reduce the risk and favour specialisation of resource use. As a consequence of reduced decision time, specialists also tend to forage more efficiently than generalists, through a search image formation on a given food type (Dukas and Kamil 2001; Terraube et al. 2011, 2014). We found a clear difference in degree of specialisation between highly heritable colour morphs (h2 = 70–92%; Brommer et al. 2005; Gasparini et al. 2009; Karell et al. 2011a), which implies that there can be a strong genetic component in diet specialisation and thereby potentially also in the sensory ecology of the tawny owl colour morphs (but see Galván and Møller 2011). Future studies should aim at investigating if the colour morphs respond differently to varying types of stimuli, (prey sound, visual cues) and whether the hunting strategies and activity rhythms differ between colour morphs.
In boreal environments, such as in Fennoscandia, small mammals can reach extremely high densities in vole peak years, which occur in 3–4-year intervals, with years of very low densities in the low phases of the vole cycle (Korpimäki et al. 2005). These cyclic food conditions limit the reproduction of tawny owls in this environment as in low vole cycle phases tawny owls often skip breeding (Karell et al. 2009a). In general, the grey tawny owls are more common in dry and cold areas (Galeotti and Cesaris 1996; PK et al. unpublished data), and they are adapted to resist snow-rich and cold winters (Karell et al. 2011a), presumably because they have lower energy requirements in extremely cold conditions (Piault et al. 2009; Karell et al. 2011a; Koskenpato et al. 2016, 2020). In concordance with this, the life span of the grey morph is indeed longer than that of the brown morph at the Northern limit of the species range (Brommer et al. 2005).
Until the late 1990s, the grey morph was clearly dominating in boreal populations (c. 70–80% of breeding owls), but milder winters have improved the survival of the brown morph and enabled it to increase in frequency in northern populations (Karell et al. 2011a). Simultaneously, vole cycles have been found to become irregular (Hörnfeldt et al. 2005; Brommer et al. 2010; Cornulier et al. 2013). These irregularities in vole cycles are likely to be more disadvantageous for the mammal-specialised grey morph compared to the generalist brown morph, since the grey morph appears to be more dependent on recurring cyclic small mammal peaks for reproduction compared to the brown morph. Brown morph parents also produce offspring in better condition than the grey morph irrespective of small mammal density (Morosinotto et al. 2020). Thereby, the brown morph may have a reproductive advantage over the grey. A simultaneous benefit of warmer winters, which reduces over-winter survival selection against the brown morph, is likely to reinforce the increase of the brown morph in more northerly environments. Therefore, if winters become milder and the small mammal abundance peaks become more irregular or absent, there is a risk that the mammal-specialised grey morph will decline in frequency, overridden by its brown generalist conspecific.
Data availability
All data are deposited in the Dryad Data Repository (https://doi.org/10.5061/dryad.3j9kd51gp).
Code availability
The R code used for statistical analyses for this study is available from the corresponding author on request.
References
Almasi B, Roulin A, Korner-Nievergelt F, Jenni-Eiermann S, Jenni L (2012) Coloration signals the ability to cope with elevated stress hormones: effects of corticosterone on growth of Barn Owls are associated with melanism. J Evol Biol 25:1189–1199
Bernays EA, Singer MS, Rodrigues D (2004) Foraging in nature: foraging efficiency and attentiveness in caterpillars with different diet breadths. Ecol Entomol 29:289–297
Bernays EA (1998) The value of being a resource specialist: behavioural support for a neural hypothesis. Am Nat 151:451–464
Bjärvall A, Ullström S (2010) Suomen nisäkkäät. Helsinki, Otava
Brommer JE, Ahola K, Karstinen T (2005) The colour of fitness: plumage coloration and lifetime reproductive success in the tawny owl. Proc R Soc Lond B 272:935–940
Brommer JE, Karell P, Aaltonen E, Ahola K, Karstinen T (2015) Dissecting direct and indirect parental effects on reproduction in a wild bird of prey: dad affects when but not how much. Behav Ecol Sociobiol 69:293–302
Brommer JE, Karell P, Pihlaja T, Painter J, Primmer C, Pietiäinen H (2003) Ural owl sex allocation and parental investment under poor food conditions. Oecologia 137:140–147
Brommer JE, Kokko H, Pietiäinen H (2000) Reproductive effort and reproductive values in periodic environments. Am Nat 155:454–472
Brommer JE, Pietiäinen H, Ahola K, Karell P, Karstinen T, Kolunen H (2010) The return of the vole cycle in southern Finland refutes the generality of the loss of cycles through “climatic forcing”. Glob Change Biol 78:577–586
Brommer JE, Pietiäinen H, Kolunen H (2002) Reproduction and survival in a variable environment: Ural owls (Strix uralensis) and the three-year vole cycle. Auk 119:544–550
Cornulier C, Yoccoz NG, Bretagnolle V et al (2013) Europe-wide dampening of population cycles in keystone herbivores. Science 340:63–66
Da Silva A, van den Brink V, Emaresi G, Luzio E, Bize P, Dreiss AN, Roulin A (2013) Melanin-based colour polymorphism signals aggressive personality in nest and territory defence in the tawny owl (Strix aluco). Behav Ecol Sociobiol 67:1041–1052
Dreiss AN, Antoniazza S, Burri R, Fumagalli L, Sonnay C, Frey C, Goudet J, Roulin A (2011) Local adaptation and matching habitat choice in female barn owls with respect to melanic coloration. J Evol Biol 25:103–114
Ducrest AL, Keller L, Roulin A (2008) Pleiotropy in the melanocortin system. coloration and behavioural syndromes. Trends Ecol Evol 23:502–510
Dukas R, Kamil AC (2001) Limited attention: the constraint underlying search image. Behav Ecol 12:192–199
Dukas R, Real LA (1991) Learning foraging tasks by bees: A comparison between social and solitary species. Anim Behav 42:269–276
Durell SE (2000) Individual feeding specialisation in shorebirds: population consequences and conservation implications. Biol Rev 75:503–518
Egan SP, Funk DJ (2006) Individual advantages to ecological specialization: insights on cognitive constraints from three conspecific taxa. Proc R Soc Lond B 273:843–848
Emaresi G, Bize P, Altwegg R, Henry I, van den Brink V, Gasparini J, Roulin A (2014) Melanin-specific life-history strategies. Am Nat 183:269–280
Emaresi G, Ducrest A-L, Bize P, Richter H, Simon C, Roulin A (2013) Pleiotropy in the melanocortin system: expression levels of this system are associated with melanogenesis and pigmentation in the tawny owl (Strix aluco). Mol Ecol 22:4915–4930
Fisher RA (1958) The genetical theory of natural selection, 2nd edn. Dover Publications, New York
Ford EB (1945) Polymorphism. Biol Rev 20:73–88
Francis CM, Saurola P (2004) Estimating components of variance in demographic parameters of tawny owls, Strix aluco. Anim Biodivers Conserv 27(1):489–502
Galeotti P, Cesaris C (1996) Rufous and grey colour morphs in the Italian tawny owl: geographical and environmental influences. J Avian Biol 27:15–20
Galeotti P, Rubolini D (2003) The niche variation hypothesis and the evolution of colour polymorphism in birds: a comparative study of owls. nightjars and raptors. Biol J Linn Soc 82:237–248. https://doi.org/10.1111/j.1095-8312.2004.00355.x
Galván I, Møller AP (2011) Brain size and the expression of pheomelanin-based colour in birds. J Evol Biol 24:999–1006
Gasparini J, Bize P, Piault R, Kazumasa W, Blount J, Roulin A (2009) Strength and cost of mounting an immune response are associated with a heritable melanin-based color trait in female tawny owls. J Anim Ecol 78:608–616
Hörnfeldt B, Hipkiss T, Eklund U (2005) Fading out of vole and predator cycles? Proc R Soc Lond B 272:2045–2049
Juutilainen T (1998) Alueellisten erojen, poikasmäärän ja pesinnän ajankohdan vaikutus viirupöllön pesäpoikasajan ravintoon. MSc thesis, University of. Helsinki
Karell P (2007) Short- and long-term consequences of food resources on Ural owl Strix uralensis reproduction. University of Helsinki, PhD-thesis http://ethesis.helsinki.fi
Karell P, Ahola K, Karstinen T, Kolunen H, Siitari H, Brommer JE (2011b) Blood parasites mediate morph-specific maintenance costs in a colour polymorphic wild bird. J Evol Biol 24:1783–1792
Karell P, Ahola K, Karstinen T, Valkama J, Brommer JE (2011a) Climate change drives microevolution in a wild bird. Nat Commun 2:208
Karell P, Ahola K, Karstinen T, Zolei A, Brommer JE (2009a) Population dynamics in a cyclic environment: consequences of cyclic food abundance on tawny owl reproduction and survival. J Anim Ecol 78:150–162
Karell P, Bensch S, Ahola K, Asghar M (2017) Pale and dark morphs of tawny owls show different patterns of telomere dynamics in relation to disease status. Proc R Soc B 284:20171127
Karell P, Pietiäinen H, Siitari H, Pihlaja T, Kontiainen P, Brommer JE (2009b) Parental allocation of additional food to own health and offspring growth in a variable environment. Can J Zool 87:8–19
Kekkonen J, Kolunen H, Pietiäinen H, Karell P, Brommer JE (2008) Tawny owl reproduction and offspring sex ratios under variable food conditions. J Ornithol 149:59–66
Korpimäki E, Hakkarinen H (2012) The Boreal owl. Ecology, behaviour and conservation of a forest-dwelling predator. Cambridge University Press, New York
Korpimäki E, Norrdahl K, Huitu O, Klemola T (2005) Predator-induced synchrony in population oscillations of coexisting small mammal species. Proc R Soc B 272:193–202
Koskenpato K, Ahola K, Karstinen T, Karell P (2016) Is the denser contour feather structure in pale grey than in pheomelanic brown tawny owls (Strix aluco) an adaptation to cold environments? J Avian Biol 47:1–6
Koskenpato K, Lehikoinen A, Lindstedt-Kareksela C, Karell P (2020) Gray tawny owls are more cryptic than brown ones in snowy landscapes in a resident colour polymorphic bird. Ecol Evol (published online, https://doi.org/10.1002/ece3.5914)
Lehikoinen A, Ranta E, Pietiäinen H, Byholm P, Saurola P, Valkama J, Huitu O, Henttonen H, Korpimäki E (2011) The impact of climate and cyclic food abundance on the timing of breeding and brood size in four boreal owl species. Oecologia 165:349–355. https://doi.org/10.1007/s00442-010-1730-1
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609
Majerus MEN (1998) Melanism: Evolution in action. Oxford University Press, Oxford
Mikkola H (1983) Owls of Europe. T and AD Poyser, Calton
Millon A, Petty SJ, Little B, Gimenez O, Cornulier T, Lambin X (2014) Dampening prey cycle overrides the impact of climate change on predator population dynamics: a long-term demographic study on tawny owls. Glob Change Biol 20:1770–1781
Morosinotto C, Ahola K, Karstinen T, Aaltonen E, Brommer JE, Lindqvist A, Karell P (2020) Fledging mass is color morph specific and affects local recruitment in a wild bird. Am Nat 196:609–619
Partridge L, Green P (1985) Intraspecific feeding specializations and population dynamics. In: Sibly RM, Smith RH (eds) Behavioural ecology: the ecological consequences of adaptive behaviour. Blackwell Scientific, Oxford, pp 207–226
Passarotto A, Parejo D, Penteriani V, Avilés JM (2018) Colour polymorphism in owls is linked to light variability. Oecologia 187:61–73
Petty SJ (1999) Diet of tawny owls (Strix aluco) in relation to field vole (Microtus agrestis) abundance in a conifer forest in northern England. J Zool 248:451–465
Piault R, Gasparini J, Bize P, Jenni-Eiermann S, Roulin A (2009) Pheomelanin-based coloration and the ability to cope with variation in food supply and parasitism. Am Nat 174:548–556
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria https://www.R-project.org/
Roulin A (2004) Covariation between plumage colour polymorphism ad diet in the Barn owl Tyto alba. Ibis 146:509–517
Roulin A, Ducret B, Ravussin P-A, Altwegg R (2003) Female colour polymorphism covaries with reproductive strategies in the tawny owl Strix aluco. J Avian Biol 34:393–401
Roulin A, Mangels J, Wakamatsu K, Bachmann T (2013) Sexually dimorphic melanin-based colour polymorphism, feather melanin content, and wing feather structure in the barn owl (Tyto alba). Biol J Linn Soc 109:562–573. https://doi.org/10.1111/bij.12078
San-José LM, Séchaud R, Schalcher K et al (2019) Differential fitness effects of moonlight on plumage colour morphs in barn owls. Nat Ecol Evol 3:1331–1340
Sasvári L, Hegyi Z, Csörgõ T, Hah I (2000) Age-dependent diet change, parental care and reproductive cost in tawny owls Strix aluco. Acta Oecol 21:267–275
Schenk K (2018) Värimuodon vaikutus lehtopöllön pesäpaikan valintaan (The effect of plumage coloration on territory choice of tawny owls). University of Helsinki, MSc thesis
Sherry TW (1990) When are birds dietarily specialized? Distinguishing ecological from evolutionary approaches. In: Morrison ML, Ralph CJ, Verner J, Jehl JR Jr (eds) Avian foraging: theory, methodology and applications. Studies in Avian Biology, vol 13. Allen press, Lawrence, pp 337–352
Siivonen L, Sulkava S (2002) Pohjolan nisäkkäät. Helsinki, Otava
Simpson EH (1949) Measurement of diversity. Nature 163:688
Sunde P, Bølstad MS, Møller JD (2003) Reversed sexual dimorphism in tawny owls, Strix aluco, correlates with duty division in breeding effort. Oikos 101:265–278
Svensson E, Sinervo B, Comendant T (2001) Density-dependent competition and selection on immune function in genetic lizard morphs. P Natl Acad Sci USA 98:12561–12565
Terraube J, Arroyo B, Madders M, Mougeot F (2011) Diet specialisation and foraging efficiency under fluctuating vole abundance: a comparison between generalist and specialist avian predators. Oikos 120:234–244
Terraube J, Guixé D, Arroyo B (2014) Diet composition and foraging success in generalist predators: are specialist individuals better foragers? Basic Appl Ecol 15:616–624
van den Brink V, Dreiss AN, Roulin A (2012) Melanin-based coloration predicts natal dispersal in the barn owl. Tyto alba. Anim Behav 84:805–812
Wilson DS, Yoshimura J (1994) On the coexistence of generalists and specialists. Am Nat 144:692–707
Whitfield DP (1990) Individual feeding specialisations in wintering Turnstone Arenaria interpres. J Anim Ecol 59:193–211
Woo KJ, Elliott KH, Davidson M, Gaston AJ, Davoren GK (2008) Individual specialization by a generalist marine predator reflects specialization in foraging behaviour. J Anim Ecol 77:1082–1091
Acknowledgements
We would like to thank two anonymous reviewers and associate editor Peter A. Bednekoff for constructive comments that helped improve the manuscript. Hannu Pietiäinen kindly helped with identifying prey in the nest material and shared his prodigious knowledge of Strix owl diet and ecology. We also thank Kari Ahola, Teuvo Karstinen, Chiara Morosinotto, Ruslan Gunko and Kati Schenk for the help in monitoring the nests and capturing the tawny owls. Arianna Passarotto provided insightful comments on a previous draft of the manuscript.
Funding
Open Access funding provided by Novia University of Applied Sciences. This work was supported by the Academy of Finland (projects 309992 and 314108 to PK), Jenny and Antti Wihuri foundation (to KaK) and Societas pro Fauna et Flora Fennica (to KiK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The procedures required to collect the data described in this work include capturing and marking of individual owls by ringing, and measuring their morphology, which all fall under the ringing licenses of the authors, as provided by the relevant local authorities. The small mammal abundance were estimated following the guidelines by the Finnish Animal Experiment Board and the Finnish Ministry of the Environment.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by P. A. Bednekoff
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 24 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karell, P., Kohonen, K. & Koskenpato, K. Specialist predation covaries with colour polymorphism in tawny owls. Behav Ecol Sociobiol 75, 45 (2021). https://doi.org/10.1007/s00265-021-02986-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-021-02986-6