Abstract
The horizontal size of the exposed depigmented sclera in Caucasians has been previously suggested to be sexually dimorphic, and the significance of this phenomenon remains unclear. Here we build on a previous study and extend it by (i) examining sex differences in other measures of ocular morphology and (ii) exploring the link between eye morphology and biometric markers of facial attractiveness. We used facial photographs of 100 Caucasians (50 men) from Eastern-Central Europe and digitally measured four ocular features. Eye measurements were tested for sex differences and associations with morphometric data on facial averageness and sexual shape dimorphism. We found that sclera surface is more horizontally exposed in men, even though the total surface area is similar in both sexes. We also found that eye fissures are rounder (less rectangular) in women than in men and that irises are brighter in women. We did not find any relationship between the examined eye features and two aspects of facial attractiveness: facial averageness and sexual dimorphism in facial shape. Despite being sexually dimorphic, eye features may be loosely linked with the development of facial sexual ornamentation. The role of sexual selection in the evolution of the observed phenomena is disputable.
Significance statement
It is often argued that because of their physical appearance, human eyes are crucial to interpersonal and social interactions. In many aspects, however, the significance of the human eye architecture is unclear. In this study, we examine sex differences in eye morphology and explore the link between ocular features and biometric measures of facial attractiveness in Caucasian men and women. We found that despite being sexually dimorphic, eye features may be loosely linked with biometric markers of facial attractiveness. We argue that the role of sexual selection in the evolution of the observed sex differences is disputable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All great apes differ markedly from other mammals, including other primates, in presenting a range of functional and morphological adaptations for increased reliance on vision, such as a less protruding snout and more exposed eyeballs (Emery 2000). The greater importance of vision is shown by the more varied ways in which great apes actively use the gaze of others in social contexts; for example, eye contact and gaze alternation in great apes have been observed to play a role in bonding (de Waal and van Roosmalen 1979; Silk 2002), communication (Gómez 1996) and competitive foraging (Bräuer et al. 2005, Kano and Call 2014; Hall et al. 2017). This great ape trend is further accentuated in humans (Homo sapiens), who rely on visual inputs to an even greater extent and make use of eyes as cues in a wider array of socially relevant functions (Kleinke 1986).
Human eyes are themselves a highly salient visual stimulus through a combination of features that give rise to a highly conspicuous morphology that enhances the perception of gaze by others. An influential study by Kobayashi and Kohshima (1997; see also Kobayashi and Kohshima 2001) compared the ocular morphology of humans and 87 other primate species and concluded that human eyes were unique in having a larger width-to-height ratio (WHR), larger surface area of the visible sclera (SSI), and an extremely depigmented sclera. The supposed morphological uniqueness and resulting unique conspicuousness of the human eye (though see Perea-García et al. 2019) resulted in several proposals to explain the adaptive origins of the peculiar morphology of the human eye.
For instance, existing data suggest that wider eyes minimized the effort required to have a horizontally ample range of vision, which could have been beneficial when hominin ancestors moved from forested areas to the savannah (Kobayashi and Kohshima 2001; see also Susskind et al. 2008 who found that changes in the eye aperture size affect sensory acquisition). Eye morphology may also promote cooperation within a group (Haley and Fessler 2005; Bateson et al. 2006; Ernest-Jones et al. 2011; Krátký et al. 2016; see also Carbon and Hesslinger 2011 for a revision of Bateson et al.’s 2006 paradigm) and establishing eye contact may assist cooperation (Behrens and Kret 2019). Similarly, a large and depigmented sclera may make it easier for conspecifics to infer intentions in cooperative tasks (Kobayashi and Kohshima 2001; Tomasello et al. 2007; Perea-García et al. 2017) and, through eye contact, establish, reinforce and negotiate social bonds (Kobayashi and Hashiya 2011). Conspicuous eye morphology together with surrounding facial features may also facilitate ostensive communication, whereby the signaller makes explicit his or her communicative intention to the receiver (Csibra et al. 2008; Tylén et al. 2012); similarly, the visibility of the white of the eye and changes of colour in the depigmented sclera (e.g. increased redness after crying) may help conspecifics infer one’s emotional state (Whalen et al. 2004; Poggi et al. 2009; Provine et al. 2013). Finally, the condition of the depigmented sclera has been found to be a cue of an individual’s health, age and attractiveness (Tomasello et al. 2007; Provine et al. 2013; Russell et al. 2014), which are significant attributes in human mate choice (e.g. Buss et al. 1989).
Importantly, sexual preferences, via sexual selection, may result in the evolution of conspicuous sexually dimorphic traits, even without any direct survival advantages (Andersson 1994). Readily apparent sexual dimorphism has been described for many anthropoid primate species in canine and body size, and pelage colour. Dimorphic features on the face, however, are much rarer, with the notable exceptions of flanges in orangutans, hair in humans and noses in proboscis monkeys (Plavcan 2001). In humans, multi-trait facial features such as sexual dimorphism in facial shape along with facial averageness have been shown to affect partner choice (Rhodes et al. 2005; Danel et al. 2016). As such, they have been investigated in relation to attractiveness as a potential index of developmental stability and health, and as costly, androgen-mediated secondary sexual traits in males (review: Little et al. 2011). The individual contribution of simpler morphological traits to these well-studied features remains to be investigated.
A potential link between scleral morphology and human sexual selection was first noted in our previous study (Danel et al. 2018b). Using a large sample of almost 600 men and women from four self-identified racial backgrounds (Ma et al. 2015), we analysed variation in sclera size as measured with the sclera size index (SSI; Kobayashi and Kohshima 1997). The study found that the size of the exposed sclera was similar in men and women self-identifying as Asian, Black and Latino but not in those self-identifying as White, where SSI was sexually dimorphic, and men had significantly more exposed sclera than women (Danel et al. 2018b). We discussed these findings with reference to the theory that specific ecological conditions intensified sexual selection in ancestral Caucasian populations of north-eastern and central Europe (see Frost (2006, 2014)). Lower reliance on female food gathering and, conversely, higher reliance on male provisioning, together with higher male mortality due to longer hunting distances, may have skewed the operational sex ratio towards a surplus of women and increased the costs of polygyny for men, thus reducing a man’s ability to support more than one mate and her offspring. In such an environment, sexual selection might have promoted the evolution of novel, unusual traits such as eye colour diversity (Frost 2006, 2014).
While iridial polymorphisms in humans could be explained by invoking sexual selection (Frost 2006, 2014), possibly enabled by changes in socioenvironmental factors in our ancestors resembling those of domesticates today (Negro et al. 2017), sexual selection mechanisms may have also affected the evolution of other aspects of ocular morphology like the sclera, the contrast between the sclera and iris, or eye shape.
In the current work, we further explored sex differences in ocular morphology in north-eastern and central European Caucasians, represented by Czechs, using a different sample than Danel et al. (2018b). Our main aim was to confirm sexual dimorphism in eye morphology and carry out exploratory analyses with a view to identifying its adaptive nature (or lack thereof). Even though the factors driving the emergence of population-specific traits in humans are a current topic of discussion, one of the most prominent hypotheses proposes that sexual selection has played an important role (Darwin 1887; van den Berghe and Frost 1986; Desmond and Moore 2010). The fact that the trait we previously identified as population-specific was also sexually dimorphic suggested a role for sexual selection. Therefore, in the present study, we wanted to
-
i.
Replicate our results on sexual dimorphism in SSI and examine sex differences related to other measures of eye fissure and scleral characteristics;
-
ii.
Study the link between eye morphology and morphological markers of facial attractiveness, namely, sex typicality (sexual dimorphism) and averageness.
Material and Methods
Facial photographs
We used facial portrait photographs of 100 individuals from the Czech Republic, collected during 2016. The sample consisted of 50 women (age: Mean ± SD = 23.64 ± 4.33, range: 19–36) and 50 men (age: Mean ± SD = 24.04 ± 3.92, range: 19–34). All participants were told to assume neutral, non-smiling expressions and not to use any facial cosmetics or other facial adornments. They were then seated in front of a white background and photographed with a digital camera (Canon 6D equipped with fixed zoom 85-mm lens) from a distance of 1.5 m using a studio electronic flash and a reflection screen. When taking portrait images, we followed the methodological recommendations suggested by Třebický et al. (2016). All photos were cropped to keep the eyes always horizontal and at the same height with a standard length of the neck visible.
Measurements
To minimize observer bias, blinded methods were used. For our analyses, we used existing materials (photographs) collected for previous studies. The ocular and facial measurements were provided independently by ZL and KK, who were blind to the actual research hypotheses at the time of data collection.
Eye measurements
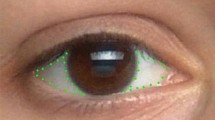
We used the measurements of ocular morphology previously introduced by Kobayashi and Kohshima (2001)—WHR (width to height ratio of the eye outline) and SSI (sclera size index = width of the exposed eyeball to the iris diameter), which we complemented with a new pixel-based measure that better captures the overall surface of visible sclera size, SSR. SSR, sclera surface ratio (SSR = ((total surface of the visible eyeball − surface of the iris with the pupil)/total surface of the visible eyeball) × 100), is the ratio of the surface of the visible sclera to the total surface of the visible eyeball. In other words, SSR reflects the percentage of the total visible eyeball surface that is occupied by the sclera. While the former measure, SSI, can be understood as a rough index of horizontally exposed sclera based on simple measurements of the iris diameter and width of visible eyeball, our new measure, SSR, is a function of the actual visible surfaces of the sclera, iris with pupil and eyeball and thus a more accurate measure of how much sclera is actually exposed. Additionally, we used RIL (relative iris luminance) developed by Perea-Garcia et al. (2017, 2019) as a measure of the relative contrast in luminance between the sclera and iris. All indices were calculated on averaged measurements obtained individually for the left and right eye. All linear and surface measurements were taken using ImageJ, version: 1.52j (Schneider et al. 2012). These four measures are presented in Fig. 1.
Schematic representation of the eye measurements used in this study. WHR consists in dividing (a) by (b); SSI is the division of (c) by (d); SSR is the division of (f–e) by (f). Picture retrieved from https://www.publicdomainpictures.net/en/view-image.php?image=130325&picture=human-eye and modified
Geometric morphometrics of the face
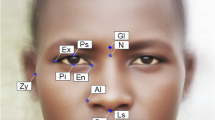
We applied a geometric morphometric (GM) approach to acquire information about the shape of human faces. Altogether, 72 landmarks were identified on each face, using tpsDig2 software (version 2.31), from which 36 points were labelled a posteriori as semi-landmarks (the same configuration was used in our previous work, e.g. Danel et al. 2016, 2018a ; Kleisner et al. 2019; see also Supplementary materials for the position of landmarks and semi-landmarks on a face). While landmarks are homologous points that are unambiguously identified on each particular specimen, semi-landmarks serve to catch the remaining shape information which is not describable by true landmarks, such as various facial curvatures. All landmark configurations were subjected to the generalized Procrustes analysis using the “gpagen” function of the geomorph package in R (Adams and Otárola-Castillo 2013). This procedure translated all objects to their origin, standardized their size and optimized their rotation until the distances between the coordinates of the corresponding points were minimized. Semi-landmarks were allowed to slide along tangents to the curve to minimize the bending energy between each specimen and the Procrustes mean configuration. Procrustes residuals were used for calculating the measures of averageness and sexual shape dimorphism.
Averageness (AVRGN) was measured as a Procrustes distance of each face from the average face in the set. This was done separately for the faces of men and women. The higher the number, the more distinct (less average) is the face.
To measure the degree of sexual dimorphism in facial shape (SShD), we calculated mean shape separately for male and female configurations. Subsequently, we projected each facial configuration onto the vector defined by the axis of sex difference between the mean male configuration and the mean female configuration (Valenzano et al. 2006; Mitteroecker et al. 2015). Along the axis that connected the two means, the position of an individual face was defined by its degree of geometric sexual dimorphism, i.e. the degree of morphological masculinity/femininity.
Statistical analysis
The normality of the data was tested with a Shapiro-Wilk test. The analysis showed that all variables were normally distributed (Table 2), with the exception of AVRGN in men’s faces. However, the values of skewness (Sk) and kurtosis (Ku) were relatively low (AVRGN: Sk = 0.80, Ku = 0.28), indicating only a minor deviation from the normal distribution. Therefore, having a balanced number of men and women in the sample, in further analyses we assumed that all the variables in both sexes were normally distributed and we used parametric methods. Consequently, differences between men and women in the sclera measurements were tested using a two-sample t test (in all tests, the assumption of homogeneity of variance was met). The associations between the eye and GM measurements were analysed with Pearson correlation coefficients. Effect sizes for t tests were calculated using the “effsize” R package, and raincloud plots were prepared according to Allen et al. (2019). We additionally used a sequential Bonferroni-Holm method (Holm 1979) to control for unintentional inflation of Type I error (i.e. an incorrect rejection of a true null hypothesis). Being a relatively liberal method, the Bonferroni-Holm procedure also protects from accepting false null hypotheses (Type II error), which could be a problem in exploratory studies such as ours (e.g. Perneger 1998; Nakagawa and Cuthill 2007). Where necessary, the obtained results were discussed with reference to this method. Analyses were carried out in STATISTICA, version 12 (data analysis software system, www.statsoft.com) and in R, version 3.6.0 (R Core Team 2019).
Results
Descriptive statistics for the eye GM measurements are presented in Table 1.
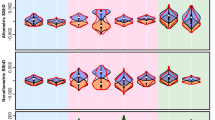
Men, compared with women, had statistically significantly higher values of SSI and WHR and lower values of RIL (Table 2, Fig. 2). In other words, men had more horizontally exposed depigmented sclera and wider (more rectangular) eye fissures as well as more contrasting irises when compared with the surrounding sclera. The difference between men and women in SSR values was statistically non-significant, indicating no sexual dimorphism in the exposed sclera surface. The effect sizes for the statistically significant differences were medium. Detailed results for the sex differences in the eye measurements are presented in Table 2 and Fig. 2. It is noteworthy that all results remained significant after applying the sequential Bonferroni-Holm correction for multiple testing (i.e. the number of multiple comparisons k = 4).
Both in men and women, none of the eye measurements was statistically significant when correlated with geometric morphometric measurements of facial averageness and sexual dimorphism. Results are presented in Table 3.
Discussion
In the present study, we investigated differences between Caucasian men and women (represented by Czechs) in several measures of ocular morphology. We also examined whether ocular features are linked to the measured facial sexual dimorphism and averageness.
Sexual dimorphism in eye morphology
We observed sex differences in two measures of eye shape (SSI and WHR) and in the brightness of the iris in relation to the sclera (RIL). Having higher values of SSI and WHR, men, compared with women, had more horizontally exposed depigmented sclera and wider eye fissures in general, despite a similar surface area of the visible sclera (SSR).
Our results on SSI are in line with the previous findings reported by Danel et al. (2018b), who found that SSI is sexually dimorphic in Caucasians, using a large multi-ethnic dataset with self-identified racial categories. The current analysis performed on data from a homogenous population confirms that the horizontal dimension of the white sclera is sexually dimorphic in Caucasians. Furthermore, the results regarding WHR support the findings from the existing facial metric studies. For instance, Hajnis et al. (1994) investigated differences between the North-American Caucasians, African-Americans and Chinese in the eye fissure index. This index is a measure of the palpebral fissure and is calculated as a ratio between the height and width of the eye, so it can be interpreted as the inverse of WHR used in the current study. In their original work, Hajnis et al. (1994) reported the highest values of the index for Caucasian men and women (i.e. rounder eyes) when compared with the two other ethnic groups within the respective sex. Although sex differences in the index were not formally tested by those authors, the data reported in their article indicate that men have in general wider eye fissures than women, and these differences are meaningful in all the three racial-ethnic groups (t test: Caucasian: t(df:101) = 4.29, p < 0.0001, d = 0.85; African-Americans: t(df:98) = 2.612, p = 0.01, d = 0.52; Chinese t(df:58) = 1.95, p = 0.056; d = 0.50). Interestingly, the effect size analysis confirms that sex differences in the eye fissure index are particularly large for Caucasians. Using a different sample of Caucasians and showing that the elliptical space between two eyelids is sexually dimorphic and wider in men, we replicated Hajnis et al.’s (1994) original findings.
Larger horizontal dimensions of the exposed sclera (i.e. SSI) and eye fissure (i.e. WHR), however, do not mean that the surface of the sclera is also sexually dimorphic. The surface area of a geometrical figure is a complex parameter that may be held constant in figures of different shapes. Therefore in our analysis, we used detailed pixel-based measurements of the eye surface and calculated how much of the whole visible eyeball area is taken up by the sclera. As noted above, we found no statistically significant differences in the visible sclera surface areas between men and women—i.e. the relative surface of the exposed sclera as measured by SSR was not sexually dimorphic. This result implies that SSI and WHR should be interpreted with caution as a measure of the size (i.e. surface area) of the exposed sclera in human and comparative studies, since interrelations between eye shape, visible eyeball and sclera surfaces may be complex.
We also studied sex differences in the contrast between the iris and sclera, i.e. relative iris luminance. We found that women had significantly higher values of RIL than men. This result implies that women’s irises are less contrasting with the surrounding sclera. While this could decrease the conspicuousness of the iris, brighter irises may be more noticeable because their brightness is concentrated within a narrower band of the visible spectrum. “Pure” colours are unusual in nature and almost always serve to attract attention, either as a warning coloration or to attract a mate (cf. Negro et al. 2017). Furthermore, a brighter iris increases the contrast with the pupil, facilitating the perception of changes in pupil size, which are known to reliably reflect arousal and emotionality (e.g. Kret and De Dreu 2019). To our knowledge, sexual dimorphism in biometrically quantified human iris luminosity has not been previously reported. In this regard, our study is the first to show that sexual dimorphism, at least in Caucasians, not only is limited to the geometric properties of the eye (i.e. SSI and WHR) but can also manifest itself in other physical characteristics, such as relative iris luminance.
Correlation with facial attractiveness markers
The second goal of our study was to examine the correlation between the scleral characteristics and widely acknowledged biometric markers of facial beauty—individual expressions of facial sexual dimorphism and averageness. These two multi-trait facial features are intensively studied in the context of biologically based human mate choice (reviewed by, e.g. Little et al. 2011). They are considered markers of physical attractiveness and play a role in shaping patterns of facial preferences (reviews: Kościński 2007; Little et al. 2011; but see Jones and Jaeger 2019 postulating that this link may be weak) and affecting real partner choice and mating decisions (Rhodes et al. 2005; Danel et al. 2016). Both facial dimorphism and averageness have been suggested as indicators of several aspects of biological quality such as heterozygosity, health and immunocompetence; however, in the light of mounting evidence from more recent studies, the significance of these links is disputable (Scott et al. 2013; Foo et al. 2017; Cai et al. 2019; Zaidi et al. 2019).
Our study showed that neither averageness nor facial sexual dimorphism was correlated with the examined characteristics of eye morphology. The lack of a statistically significant association between sexually dimorphic ocular features and expression of sexual dimorphism is in line with several previous studies on facial morphogenesis. Multiple genetic, epigenetic as well as functional, environmental and social factors affecting the development of human faces (Enlow 1990; Liu et al. 2012; Claes et al. 2014; Hallgrimsson et al. 2014; Sheehan and Nachman 2014) may result in tenuous correlations between facial features (Wang et al. 2013) even if they are seemingly similar in function and biological significance (Danel et al. 2018a). Moreover, the lack of a correlation with facial attractiveness markers suggests that the ocular features under consideration are loosely linked with the development of facial sexual ornaments. This may also indicate that the differences we report above are not related to advertising facial beauty, which may open the door to alternative explanations of their nature.
Does sexual dimorphism in eye morphology have adaptive significance?
A potential specificity to Caucasians of the sex differences in SSI (see Danel et al. (2018b)) and a high magnitude of sexual dimorphism in WHR observed particularly in Caucasians (Hajnis et al. 1994; and discussed above) may suggest that some geographically restricted ecological conditions contributed to the observed sexual dimorphism. However, the non-significant correlations between the examined eye features and facial markers of attractiveness point out that mechanisms other than sexual selection may have contributed to the development of these sex differences.
Individual heterogeneity in biotic interactions or responses to abiotic conditions, which lead to phenotypical variation within species and populations (Dall et al. 2012), may be one of such mechanisms. It has been proposed that such niche specialisations may be a driving force of the evolution of ecological sexual dimorphism independently of sexual selection (Darwin 1871; Selander 1966; Slatkin 1984; Hedrick and Temeles 1989; Shine 1989; Andersson 1994; Temeles et al. 2000). Nonetheless, on the basis of the current state of knowledge, it is difficult to identify ecological factors, divergent for men and women, that would have facilitated the evolution of sexual dimorphism in Caucasians but not in other populations. The most recent cross-cultural studies, however, showed considerable geographical variation both in the magnitude and morphological pattern of facial sexual dimorphism (Kleisner et al. 2020).
It is also possible that the observed sex differences in eye morphology may have no direct functional significance. This may occur, for instance, when the observed sexual dimorphism in the eyes simply reflects allometric relations with the different body size in men and women. In fact, cross-species correlations between SSI and body size parameters of different primates have been reported by Kobayashi and Kohshima (2001) and other research showed that allometric relations (not considered in our study) may contribute to size-dependent sexual dimorphism in human face shape (see Mitteroecker et al. 2013; Mitteroecker et al. 2015; Kleisner et al. 2020 for further discussion).
Similarly, if the ecological function of the observed sex differences is unclear, and sexual selection cannot explain it, sexual dimorphism in eye morphology may be a spandrel (see Gould and Lewontin (1979)). On this interpretation, the different eye morphology in Caucasian men and women would be just a functionless by-product of the evolution of some other, yet-to-be-determined functional features. Nonetheless, the relatively large effect size of the observed sex differences suggests substantial biological importance of our findings, calling for further exploration of potential functions of the dimorphism observed in this study.
Limitations and further research
In our study, we focused on the biometric measures of eyes and facial characteristics. Since facial expression of sexual dimorphism is one of the predictors of facial beauty (Little et al. 2011), we encourage future studies to investigate whether ocular morphology (and the sexually dimorphic features in particular) affect perceptual ratings of facial attractiveness. Furthermore, allometric and non-allometric components of sexual dimorphism (cf. Kleisner et al. 2020) in multiple aspects of the human eye should be examined in other populations and ethnic groups from various ecological backgrounds to explore the nature and confirm or disconfirm the universality of the observed phenomenon. Similarly, comparative studies involving other primate species may shed more light on the evolution of sex differences and their biological function.
Conclusions
In this study, we examined sex differences in ocular morphology in Caucasians. We found several sexually dimorphic eye features but none of them correlated with the two aspects of facial attractiveness: facial averageness and sexual dimorphism in facial shape. This suggests a minor role of sexual selection in the evolution of eye sexual dimorphism. Other, perhaps non-adaptive, processes may have contributed to the development of the observed phenomenon. Our conclusions should be evaluated in the context of the monoethnic sample of Caucasians. Both the finding about sexual dimorphism of eye morphology and the problem of its evolutionary implications should be a subject of comparative studies on primate species as well as cross-ethnic examinations of humans.
Data availability
The data that supports the findings of this study is available in the supplementary material of this article.
References
Adams DC, Otárola-Castillo E (2013) Geomorph: an r package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol 4:393–399. https://doi.org/10.1111/2041-210X.12035
Allen M, Poggiali D, Whitaker K, Marshall TR, Kievit RA (2019) Raincloud plots: a multi-platform tool for robust data visualization [version 1; peer review: 2 approved]. Wellcome Open Res 4:63. https://doi.org/10.12688/wellcomeopenres.15191.1
Andersson M (1994) Sexual Selection. Princeton University Press, Princeton, NJ
Bateson M, Nettle D, Roberts G (2006) Cues of being watched enhance cooperation in a real-world setting. Biol Lett 2:412–414. https://doi.org/10.1098/rsbl.2006.0509
Behrens F, Kret ME (2019) The interplay between face-to-face contact and feedback on cooperation during real-life interactions. J Nonverbal Behav 43:513–528. https://doi.org/10.1007/s10919-019-00314-1
Bräuer J, Call J, Tomasello M (2005) All great ape species follow gaze to distant locations and around barriers. J Comp Psychol 119:145–154. https://doi.org/10.1037/0735-7036.119.2.145
Buss DM, Darwin C, Lacaita CC et al (1989) Sex differences in human mate preferences: evolutionary hypotheses tested in 37 cultures. Behav Brain Sci 12:1–14. https://doi.org/10.1017/S0140525X00023992
Cai Z, Hahn AC, Zhang W, Holzleitner IJ, Lee AJ, DeBruine LM, Jones BC (2019) No evidence that facial attractiveness, femininity, averageness, or coloration are cues to susceptibility to infectious illnesses in a university sample of young adult women. Evol Hum Behav 40:156–159. https://doi.org/10.1016/J.EVOLHUMBEHAV.2018.10.002
Carbon CC, Hesslinger VM (2011) Bateson et al.’s (2006) Cues-of-being-watched paradigm revisited. Swiss J Psychol 70:203–210. https://doi.org/10.1024/1421-0185/a000058
Claes P, Liberton DK, Daniels K, Rosana KM, Quillen EE, Pearson LN, McEvoy B, Bauchet M, Zaidi AA, Yao W, Tang H, Barsh GS, Absher DM, Puts DA, Rocha J, Beleza S, Pereira RW, Baynam G, Suetens P, Vandermeulen D, Wagner JK, Boster JS, Shriver MD (2014) Modeling 3D facial shape from DNA. PLoS Genet 10:e1004224. https://doi.org/10.1371/journal.pgen.1004224
Csibra G, Kushnerenko E, Grossmann T (2008) Electrophysiological methods in studying infant cognitive development. In: Nelson CA, Luciana M (eds) Handbook of Developmental Cognitive Neuroscience, 2nd edn. MIT Press, Cambridge, London
Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW (2012) An evolutionary ecology of individual differences. Ecol Lett 15:1189–1198. https://doi.org/10.1111/j.1461-0248.2012.01846.x
Danel DP, Dziedzic-Danel A, Kleisner K (2016) Does age difference really matter? Facial markers of biological quality and age difference between husband and wife. HOMO 67:337–347. https://doi.org/10.1016/j.jchb.2016.05.002
Danel DP, Valentova JV, Sánchez OR, Leongómez JD, Varella MAC, Kleisner K (2018a) A cross-cultural study of sex-typicality and averageness: correlation between frontal and lateral measures of human faces. Am J Hum Biol 30:e23147. https://doi.org/10.1002/ajhb.23147
Danel DP, Wacewicz S, Lewandowski Z, Żywiczyński P, Perea-García JO (2018b) Humans do not perceive conspecifics with a greater exposed sclera as more trustworthy: a preliminary cross-ethnic study of the function of the overexposed human sclera. Acta Ethol 21:203–208. https://doi.org/10.1007/s10211-018-0296-5
Darwin C (1871) The descent of men, and selection in relation to sex. Murray, London
Desmond A, Moore J (2010) Darwin’s sacred cause: race, slavery and the quest for human origins. Penguin UK, London
de Waal FBM, van Roosmalen A (1979) Reconciliation and consolation among chimpanzees. Behav Ecol Sociobiol 5:55–66
Emery NJ (2000) The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev 24:581–604. https://doi.org/10.1016/S0149-7634(00)00025-7
Enlow DH (1990) Facial Growth, 3rd edn. W.B. Saunders, Philadelphia
Ernest-Jones M, Nettle D, Bateson M (2011) Effects of eye images on everyday cooperative behavior: a field experiment. Evol Hum Behav 32:172–178. https://doi.org/10.1016/j.evolhumbehav.2010.10.006
Foo YZ, Simmons LW, Rhodes G (2017) Predictors of facial attractiveness and health in humans. Sci Rep 7:39731. https://doi.org/10.1038/srep39731
Frost P (2006) European hair and eye color: A case of frequency-dependent sexual selection? Evol Hum Behav 27:85–103. https://doi.org/10.1016/j.evolhumbehav.2005.07.002
Frost P (2014) The puzzle of European hair, eye, and skin color. Adv Anthropol 4:78–88. https://doi.org/10.4236/aa.2014.42011
Gómez JC (1996) Ostensive behavior in great apes: The role of eye contact. In: Russon AE, Bard KA, Parker ST (eds) Reaching into thought: The minds of the great apes. Cambridge University Press, Cambridge, pp 131–151
Gould SJ, Lewontin RC (1979) The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc R Soc Lond B 205:581–598. https://doi.org/10.1098/rspb.1979.0086
Hajnis K, Farkas LG, Ngim RCK, Lee ST, Venkatadri G (1994) Racial and ethnic morphometric differences in the craniofacial complex. In: Farkas LG (ed) Anthropometry of the Head and Face, 2nd edn. Raven Press, New York, pp 201-218
Haley KJ, Fessler DMT (2005) Nobody’s watching? Subtle cues affect generosity an anonymous economic game. Evol Hum Behav 26:245–256. https://doi.org/10.1016/j.evolhumbehav.2005.01.002
Hall K, Oram MW, Campbell MW, Eppley TM, Byrne RW, de Waal FBM (2017) Chimpanzee uses manipulative gaze cues to conceal and reveal information to foraging competitor. Am J Primatol 79:1–11. https://doi.org/10.1002/ajp.22622
Hallgrimsson B, Mio W, Marcucio RS, Spritz R (2014) Let’s face it--complex traits are just not that simple. PLoS Genet 10:e1004724. https://doi.org/10.1371/journal.pgen.1004724
Hedrick AV, Temeles EJ (1989) The evolution of sexual dimorphism in animals: Hypotheses and tests. Trends Ecol Evol 4:136–138. https://doi.org/10.1016/0169-5347(89)90212-7
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Jones AL, Jaeger B (2019) Biological bases of beauty revisited: the effect of symmetry, averageness, and sexual dimorphism on female facial attractiveness. Symmetry 11:279. https://doi.org/10.3390/sym11020279
Kano F, Call J (2014) Cross-species variation in gaze following and conspecific preference among great apes, human infants and adults. Anim Behav 91:137–150. https://doi.org/10.1016/j.anbehav.2014.03.011
Kleinke CL (1986) Gaze and eye contact: a research review. Psychol Bull 100:78–100. https://doi.org/10.1037/0033-2909.100.1.78
Kleisner K, Pokorný Š, Saribay SA (2019) Toward a new approach to cross-cultural distinctiveness and typicality of human faces: the cross-group typicality/distinctiveness metric. Front Psychol 10:124. https://doi.org/10.3389/fpsyg.2019.00124
Kleisner K, Tureček P, Roberts CS, Havliček J, Valentova JV, Akoko RM, Leongómez JD, Apostol S, Varella MAC, Saribay SA (2020) How and why patterns of sexual dimorphism in human faces vary across the world. PsyArXiv 10 Feb. 2020, https://doi.org/10.31234/osf.io/7vdmb
Kobayashi H, Hashiya K (2011) The gaze that grooms: Contribution of social factors to the evolution of primate eye morphology. Evol Hum Behav 32:157–165. https://doi.org/10.1016/j.evolhumbehav.2010.08.003
Kobayashi H, Kohshima S (1997) Unique morphology of the human eye. Nature 387:767–768. https://doi.org/10.1038/42842
Kobayashi H, Kohshima S (2001) Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol 40:419–435. https://doi.org/10.1006/jhev.2001.0468
Kościński K (2007) Facial attractiveness: general patterns of facial preferences. Anthropol Rev 70:45–79. https://doi.org/10.2478/v10044-008-0001-9
Krátký J, Lang M, Shaver JH, Jerotijević D, Xygalatas D (2016) Anxiety and ritualization: can attention discriminate compulsion from routine? Commun Integr Biol 9:e1174799. https://doi.org/10.1080/19420889.2016.1174799
Kret ME, De Dreu CKW (2019) The power of pupil size in establishing trust and reciprocity. J Exp Psychol Gen 148:1299–1311. https://doi.org/10.1037/xge0000508
Little AC, Jones BC, DeBruine LM (2011) Facial attractiveness: evolutionary based research. Philos Trans R Soc B 366:1638–1659. https://doi.org/10.1098/rstb.2010.0404
Liu F, van der Lijn F, Schurmann C, Zhu G, Chakravarty MM, Hysi PG, Wollstein A, Lao O, de Bruijne M, Ikram MA, van der Lugt A, Rivadeneira F, Uitterlinden AG, Hofman A, Niessen WJ, Homuth G, de Zubicaray G, McMahon KL, Thompson PM, Daboul A, Puls R, Hegenscheid K, Bevan L, Pausova Z, Medland SE, Montgomery GW, Wright MJ, Wicking C, Boehringer S, Spector TD, Paus T, Martin NG, Biffar R, Kayser M (2012) A genome-wide association study identifies five loci influencing facial morphology in Europeans. PLoS Genet 8:e1002932. https://doi.org/10.1371/journal.pgen.1002932
Ma DS, Correll J, Wittenbrink B (2015) The Chicago face database: a free stimulus set of faces and norming data. Behav Res Methods 47:1122–1135. https://doi.org/10.3758/s13428-014-0532-5
Mitteroecker P, Gunz P, Windhager S, Schaefer K (2013) A brief review of shape, form, and allometry in geometric morphometrics, with applications to human facial morphology. Hystrix 24:59–66. https://doi.org/10.4404/hystrix-24.1-6369
Mitteroecker P, Windhager S, Müller GB, Schaefer K (2015) The morphometrics of “masculinity” in human faces. PLoS One 10:e0118374. https://doi.org/10.1371/journal.pone.0118374
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605. https://doi.org/10.1111/j.1469-185X.2007.00027.x
Negro JJ, Carmen-Blázquez M, Galván I (2017) Intraspecific eye color variability in birds and mammals: a recent evolutionary event exclusive to humans and domestic animals. Front Zool 14:53. https://doi.org/10.1186/s12983-017-0243-8
Perea-García JO, Ehlers KR, Tylén K (2017) Bodily constraints contributing to multimodal referentiality in humans: the contribution of a de-pigmented sclera to proto-declaratives. Lang Commun 54:73–81. https://doi.org/10.1016/j.langcom.2016.10.007
Perea-García JO, Kret ME, Monteiro A, Hobaiter C (2019) Scleral pigmentation leads to conspicuous, not cryptic, eye morphology in chimpanzees. P Natl Acad Sci USA 116:19248–19250. https://doi.org/10.1073/pnas.1911410116
Perneger TV (1998) What’s wrong with Bonferroni adjustments. Brit Med J 316:1236–1238. https://doi.org/10.1136/bmj.316.7139.1236
Plavcan JM (2001) Sexual dimorphism in primate evolution. Yearb Phys Anthropol 44:25–53. https://doi.org/10.1002/ajpa.10011
Poggi I, D’Errico F, Spagnolo A (2009) The embodied morphemes of gaze. Lect Notes Comput Sc 5934:34–46. https://doi.org/10.1007/978-3-642-12553-9_4
Provine RR, Nave-Blodgett J, Cabrera MO (2013) The emotional eye: red sclera as a uniquely human cue of emotion. Ethology 119:993–998. https://doi.org/10.1111/eth.12144
Rhodes G, Simmons LW, Peters M (2005) Attractiveness and sexual behavior: does attractiveness enhance mating success? Evol Hum Behav 26:186–201. https://doi.org/10.1016/J.EVOLHUMBEHAV.2004.08.014
Russell R, Sweda JR, Mauger E, Porcheron A, Mauger E (2014) Sclera color changes with age and is a cue for perceiving age, health, and beauty. Psychol Aging 29:626–635. https://doi.org/10.1037/a0036142
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Scott IML, Clark AP, Boothroyd LG, Penton-Voak IS (2013) Do men’s faces really signal heritable immunocompetence? Behav Ecol 24:579–589. https://doi.org/10.1093/beheco/ars092
Selander RK (1966) Sexual dimorphism and differential niche utilization in birds. Condor 68:113–151. https://doi.org/10.2307/1365712
Sheehan MJ, Nachman MW (2014) Morphological and population genomic evidence that human faces have evolved to signal individual identity. Nat Commun 5:4800. https://doi.org/10.1038/ncomms5800
Shine R (1989) Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q Rev Biol 64:419–461
Silk JB (2002) The form and function of reconciliation in primates. Annu Rev Anthropol 31:21–44
Slatkin M (1984) Ecological causes of sexual dimorphism. Evolution 38:622–630. https://doi.org/10.2307/2408711
Susskind JM, Lee DH, Cusi A, Feiman R, Grabski W, Anderson AK (2008) Expressing fear enhances sensory acquisition. Nat Neurosci 11:843–850. https://doi.org/10.1038/nn.2138
Temeles EJ, Pan IL, Brennan JL, Horwitt JN (2000) Evidence for ecological causation of sexual dimorphism in a hummingbird. Science 289:441–443. https://doi.org/10.1126/science.289.5478.441
Tomasello M, Hare B, Lehmann H, Call J (2007) Reliance on head versus eyes in the gaze following of great apes and human infants: the cooperative eye hypothesis. J Hum Evol 52:314–320. https://doi.org/10.1016/j.jhevol.2006.10.001
Třebický V, Fialová J, Kleisner K, Havlíček J (2016) Focal length affects depicted shape and perception of facial images. PLoS One 11:e0149313. https://doi.org/10.1371/journal.pone.0149313
Tylén K, Allen M, Hunter BK, Roepstorff A (2012) Interaction versus observation: distinctive modes of social cognition in human brain and behavior? A combined fMRI and eye-tracking study. Front Hum Neurosci 6:1–11. https://doi.org/10.3389/fnhum.2012.00331
Valenzano DR, Mennucci A, Tartarelli G, Cellerino A (2006) Shape analysis of female facial attractiveness. Vis Res 46:1282–1291. https://doi.org/10.1016/j.visres.2005.10.024
van den Berghe PL, Frost P (1986) Skin color preference, sexual dimorphism and sexual selection: a case of gene culture co-evolution? Ethn Racial Stud 9:87–113. https://doi.org/10.1080/01419870.1986.9993516
Wang MF, Otsuka T, Akimoto S, Sato S (2013) Vertical facial height and its correlation with facial width and depth: three dimensional cone beam computed tomography evaluation based on dry skulls. Int J Stomatol Occlusion Med 6:120–129. https://doi.org/10.1007/s12548-013-0089-4
Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, McLaren D, Somerville LH, McLean A, Maxwell JS, Johnstone T (2004) Human amygdala responsivity to masked fearful eye whites. Science 306:2061. https://doi.org/10.1126/science.1103617
Zaidi AA, White JD, Mattern BC, Liebowitz CR, Puts DA, Claes P, Shriver MD (2019) Facial masculinity does not appear to be a condition-dependent male ornament and does not reflect MHC heterozygosity in humans. P Natl Acad Sci USA 116:1633–1638. https://doi.org/10.1073/pnas.1808659116
Acknowledgements
We thank Zuzana Štěrbová and Šimon Pokorný for their technical assistance. We are also grateful to three anonymous reviewers for valuable feedback on the manuscript.
Funding
KK was supported by Czech Science Foundation grant number GA18-10298S. SW, MEK and PZ were supported by the Polish National Agency for Academic Exchange under Grant No. PPI/APM/2018/1/00036/U/001. Research was further supported by the European Research Council (Starting grant # 804582 to MEK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (The Institutional Review Board of Charles University Faculty of Science; protocol ref. number: 06/2017) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement of informed consent
In the current study, we used materials collected for previous studies. All photographed individuals gave informed consent to use their portraits in scientific research.
Additional information
Communicated by M. Raymond
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Danel, D.P., Wacewicz, S., Kleisner, K. et al. Sex differences in ocular morphology in Caucasian people: a dubious role of sexual selection in the evolution of sexual dimorphism of the human eye. Behav Ecol Sociobiol 74, 115 (2020). https://doi.org/10.1007/s00265-020-02894-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02894-1