Abstract
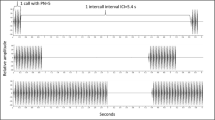
To evaluate the effects of calling site on call degradation, we broadcast synthetic advertisement calls of male gray treefrogs through forest, over open terrain, and across pond water. Calls were recorded at distances of 1, 2, 4, 8, 16, and 32 m. We varied speaker and microphone heights for a total of five elevation combinations ranging from surface level to a height of 1.5 m. We quantified structural degradation in recorded calls using “∆V,” a measure of relative sound energy in call pulses and interpulse intervals. A subset of recorded calls was used in two-speaker discrimination tests with females. Finally, we examined male selection of perch height by recording locations of calling males on ladder-like trellises positioned around the periphery of a breeding pond. We found the greatest degradation for calls broadcast through forest followed by calls transmitted across open terrain and then pond water. At relatively small source-receiver separations, elevation had only small effects on degradation. However, for separations greater than 4 m (especially through forest), elevation had a significant impact on ∆V—with calls broadcast and recorded near the substrate particularly vulnerable to degradation. Choice tests demonstrated that such levels of degradation could significantly reduce a male’s attractiveness. This may, in part, explain why males only seldom called from low rungs of trellises.








Similar content being viewed by others
References
Amézquita A, Hödl W (2004) How, when, and where to perform visual displays? The case of the Amazonian frog Hyla parviceps. Herpetologica 60:20–29
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Arak A, Eiriksson T (1992) Choice of singing sites by male bushcrickets (Tettigonia viridissima) in relation to signal propagation. Behav Ecol Sociobiol 30:365–372
Attenborough K (2002) Sound propagation close to the ground. Annu Rev Fluid Mech 34:51–82
Aylor D (1972) Noise reduction by vegetation and ground. J Acoust Soc Am 51:197–205
Barker NKS, Mennill DJ (2009) Song perch height in rufous-and-white wrens: does behaviour enhance effective communication in a tropical forest? Ethology 115:897–904
Barker NKS, Dabelsteen T, Mennill DJ (2009) Degradation of male and female rufous-and-white wren songs in a tropical forest: effects of sex, perch height, and habitat. Behaviour 146:1093–1022
Beckers OM, Schul J (2004) Phonotaxis in Hyla versicolor (Anura: Hylidae): the effect of absolute call amplitude. J Comp Physiol A 190:869–876
Bee MA, Micheyl C (2008) The cocktail party problem: What is it? How can it be solved? And why should animal behaviorists study it? J Comp Psychol 122:235–251
Bee MA, Schwartz JJ (2009) Perception by frogs in the presence of chorus-shaped noise: I. Behavioral measures of signal recognition thresholds. J Acoust Soc Am 126:2788–2801
Bee MA, Vélez A, Forester JD (2012) Sound level discrimination by gray treefrogs in the presence and absence of chorus-shaped noise. J Acoust Soc Am 131:4188–4195
Bosch J, De la Riva I (2004) Are frog calls modulated by the environment? An analysis with anuran species from Bolivia. Can J Zool 82:880–888
Brenowitz EA, Wilczynski W, Zakon HH (1984) Acoustic communication in spring peepers: environmental and behavioral aspects. J Comp Physiol A 155:585–592
Brumm H, Naguib M (2009) Environmental acoustics and the evolution of bird song. Adv Study Behav 40:1–33
Bush SL, Gerhardt HC, Schul J (2002) Pattern recognition and call preferences in treefrogs (Anura: Hylidae): a quantitative analysis using a no-choice paradigm. Anim Behav 63:7–14
Castellano S, Giacoma C, Ryan MJ (2003) Call degradation in diploid and tetraploid green toads. Biol J Linn Soc 78:11–26
Christie KJ, Schul J, Feng AS (2010) Phonotaxis to male’s calls embedded within a chorus by female gray treefrogs, Hyla versicolor. J Comp Physiol A 196:569–579
Dabelsteen T, Pedersen SB, Larsen ON (1993) Habitat-induced degradation of sound signals: quantifying the effects of communication sounds and bird location on blur ratio, excess attenuation and signal-to-noise ratio. J Acoust Soc Am 93:2206–2220
Embleton TFW (1996) Tutorial on sound propagation outdoors. J Acoust Soc Am 100:31–48
Erdtmann LK, Lima AP (2013) Environmental effects on anuran call design: what we know and what we need to know. Ethol Ecol Evol 25:1–11
Etges WJ (1987) Call site choice in male anurans. Copeia 1987:910–923
Ey E, Fischer J (2009) The “acoustic adaptation hypothesis”—a review of the evidence from birds, anurans and mammals. Bioacoustics 19:21–48
Fellers GM (1979a) Mate selection in the gray treefrog, Hyla versicolor. Copeia 1979:286–290
Fellers GM (1979b) Aggression, territoriality, and mating behaviour in North American treefrogs. Anim Behav 27:107–119
Feng AS, Ratnam R (2000) Neural basis of hearing in real-world situations. Annu Rev Psychol 51:699–725
Feng SS, Schul J (2006) Sound processing in real-world environments. In: Narins PM, Feng AS, Fay RR, Popper AN (eds) Springer handbook of auditory research: hearing and sound communication in amphibians. Springer-Verlag, New York, pp 323–350
Forrest TG (1994) From sender to receiver: propagation and environmental effects on acoustic signals. Am Zool 34:644–654
Gerhardt HC (1975) Sound pressure levels and radiation patterns of vocalizations of some North American frogs and toads. J Comp Physiol 102:1–12
Gerhardt HC (1976) Significance of two frequency bands in long distance vocal communication in the green treefrog. Nature 261:692–694
Gerhardt HC (1991) Female mate choice in treefrogs. Static and dynamic acoustic criteria. Anim Behav 42:615–635
Gerhardt HC (2005a) Acoustic spectral preferences in two cryptic species of grey treefrogs: implications for mate choice and sensory mechanisms. Anim Behav 70:39–49
Gerhardt HC (2005b) Advertisement-call preferences in diploid-tetraploid treefrogs (Hyla chrysoscelis and Hyla versicolor): implications for mate choice and the evolution of communication systems. Evolution 59:395–408
Gerhardt HC, Huber F (2002) Acoustic communication in insects and frogs: common problems and diverse solutions. University of Chicago Press, Chicago
Gerhardt HC, Klump GM (1988) Masking of acoustic signals by the chorus background noise in the green tree frog: a limitation on mate choice. Anim Behav 36:1247–1249
Gerhardt HC, Schul J (1999) A quantitative analysis of behavioral selectivity for pulse-rise time in the gray treefrog, Hyla versicolor. J Comp Physiol A 185:33–40
Gerhardt HC, Dyson ML, Tanner SD (1996) Dynamic acoustic properties of the advertisement calls of gray tree frogs: patterns of variability and female choice. Behav Ecol 7:7–18
Gerhardt HC, Tanner SD, Corrigan CM, Walton HC (2000) Female preference functions based on call duration in the gray tree frog (Hyla versicolor). Behav Ecol 11:663–669
Gerhardt HC, Martínez CC, Schwartz JJ, Marshall VT, Murphy CG (2007) Preferences based on spectral differences in acoustic signals in four species of treefrog (Anura: Hylidae). J Exp Biol 210:2990–2998
Guilford T, Dawkins MS (1991) Receiver psychology and the evolution of animal signals. Anim Behav 42:1–14
Hebets EA, Papaj DR (2005) Complex signal function: developing a framework of testable hypotheses. Behav Ecol Sociobiol 57:197–214
Höbel G, Barta T (2014) Adaptive plasticity in calling site selection in grey treefrogs (Hyla versicolor). Behaviour 151:741–754
Johnson C (1966) Species recognition in the H. versicolor complex. Tex J Sci 18:361–364
Johnson JR, Knouft JH, Semlitsch RD (2007) Sex and seasonal differences in the spatial terrestrial distribution of gray treefrog (Hyla versicolor) populations. Biol Conserv 140:250–258
Kats LB, Bucciarelli GM, Schlais DE, Blaustein AR, Han BA (2012) Ultraviolet radiation influences perch selection by a neotropical poison-dart frog. PLoS ONE 7:e51364
Kime NM, Turner WR, Ryan MJ (2000) The transmission of advertisement calls in Central American frogs. Behav Ecol 11:71–83
Klump GM, Gerhardt HC (1987) Use of non-arbitray acoustic criteria in mate choice by female gray treefrogs. Nature 326:286–288
Kuczynski MV, Vélez A, Schwartz JJ, Bee MA (2010) Sound transmission and the recognition of temporally degraded sexual advertisement signals in Cope's gray treefrog (Hyla chrysoscelis). J Exp Biol 213:2840–2850
Lang F (2000) Acoustic communication distances of a gomphocerine grasshopper. Bioacoustics 10:233–258
Lardner B, Bin Lakim M (2002) Animal communication: tree-hole frogs exploit resonance effects. Nature 420:475
Marten K, Marler P (1977) Sound transmission and its significance for animal vocalization. I. Temperate habitats. Behav Ecol Sociobiol 2:271–290
Mathevon N, Dabelsteen T, Blumenrath SH (2008) Are high perches in the blackcap Sylvia atricapilla song or listening posts? A sound transmission study. J Acoust Soc Am 114:442–449
Morton E (1975) Ecological sources of selection on avian sounds. Am Nat 109:17–34
Murphy CG (2003) The cause of correlations between nightly numbers of male and female barking treefrogs (Hyla gratiosa) attending choruses. Behav Ecol 14:274–281
Murphy CG (2008) Assessment of distance to potential mates by female barking treefrogs (Hyla gratiosa). J Comp Psychol 122:264–273
Murphy CG, Gerhardt HC (2002) Mate sampling by female barking treefrogs (Hyla gratiosa). Behav Ecol 13:472–480
Narins PM, Zelick R (1988) The effects of noise on auditory processing and behavior in amphibians. In: Fritszch B, Wilczynski W, Ryan MJ, Hetherington T, Walkowiak W (eds) The evolution of the amphibian auditory system. Wiley, USA, pp 511–536
Padgham M (2004) Reverberation and frequency attenuation in forests—implications for acoustic communication in animals. J Acoust Soc Am 115:402–410
Parris KM (2002) More bang for your buck: the effect of caller position, habitat and chorus noise on the efficiency of calling in the spring peeper. Ecol Model 156:213–224
Penna M, Marquez R, Bosch J, Crespo EG (2006) Nonoptimal propagation of advertisement calls of midwife toads in Iberian habitats. J Acoust Soc Am 119:1227–1237
Penna M, Plaza A, Moreno-Gómez FN (2013) Severe constraints for sound communication in a frog from the South American temperate forest. J Comp Physiol A 199:723–733
Pröhl H, Hödl W (1999) Parental investment, potential reproductive rates, and mating system in the strawberry dart-poison frog, Dendrobates pumilio. Behav Ecol Sociobiol 46:215–220
Ptacek MB (1992) Calling sites used by male gray treefrogs, Hyla versicolor and Hyla chrysoscelis, in sympatry and allopatry in Missouri. Herpetologica 48:373–382
Ralin DB (1968) Ecological and reproductive differentiation in the cryptic species of the H. versicolor complex (Hylidae). Southwest Nat 13:283–300
Ratnam R, Iyer N, Goense J, Feng FS (2004) Effect of reverberation on neural response to amplitude modulated signals. ARO Abstr 27:113 (#336)
Reichert MS, Gerhardt HC (2012) Trade-offs and upper limits to signal performance during close-range vocal competition in gray tree frogs Hyla versicolor. Am Nat 180:425–437
Röhr DL, Junca FA (2013) Micro-habitat influence on the advertisement call structure and sound propagation efficiency of Hypsiboas crepitans (Anura: Hylidae). J Herpetol 47:549–554
Römer H (2012) The sensory ecology of acoustic communication in insects. In: Hoy RR, Fay RR (eds) Comparative hearing: insects. Springer, New York, pp 63–96
Römer H, Lewald J (1992) High-frequency sound transmission in natural habitats: implications for the evolution of insect acoustic communication. Behav Ecol Sociobiol 29:437–444
Rose GJ, Capranica RR (1984) Processing amplitude-modulated sounds by the auditory midbrain of two species of toads: Matched temporal filters. J Comp Physiol A 154:211–219
Rose GJ, Hanson JL, Leary CJ, Graham JA, Alluri RK, Vasquez-Opazo GA (2015) Species-specificity of temporal processing in the auditory midbrain of gray treefrogs: interval-counting neurons. J Comp Physiol A 201:485–503
Runkle L (1992) Seasonal and individual variation in the vocal behavior and energetics of the gray treefrog, Hyla versicolor. Unpublished MS Thesis, University of Connecticut, Storrs CT
Ryan MJ, Cummings ME (2005) Animal signals and the overlooked costs of efficacy. Evolution 59:1160–1161
Ryan MJ, Keddy-Hector A (1992) Directional patterns of female mate choice and the role of sensory biases. Am Nat 139:S4–S35
Ryan MJ, Kime NM (2003) Selection on long distance acoustic signals. In: Simmons A, Fay R, Popper A (eds) Springer handbook of auditory research: acoustic communication. Springer Verlag, New York, pp 225–274
Ryan MJ, Sullivan BK (1989) Transmission effects on temporal structure in the advertisement calls of two toads, Bufo woodhousii and Bufo valliceps. Ethology 80:182–189
Ryan MJ, Cocroft RB, Wilczynski W (1990) The role of environmental selection in intraspecific divergence of mate recognition signals in the cricket frog, Acris crepitans. Evolution 44:1869–1872
Sabatini V, Ruiz-Miranda CR (2008) Acoustical aspects of the propagation of long calls of wild Leontopithecus rosalia. Int J Primatol 29:207–223
Schul J, Bush SL (2002) Non-parallel coevolution of sender and receiver in the acoustic communication system of treefrogs. Proc R Soc Lond B 269:1847–1852
Schwartz JJ (1987) The function of call alternation in anuran amphibians: a test of three hypotheses. Evolution 41:461–471
Schwartz JJ, Bee MA (2013) Anuran acoustic signal production in noisy environments. In: Brumm H (ed) Animal communication and noise. Springer, New York, pp 91–132
Schwartz JJ, Gerhardt HC (1995) Directionality of the auditory system and call pattern recognition during acoustic interference in the gray treefrog, Hyla versicolor. Audit Neurosci 1:195–206
Schwartz JJ, Marshall VT (2006) Forms of call overlap and their impact on advertisement call attractiveness to females of the gray treefrog, Hyla versicolor. Bioacoustics 16:39–56
Schwartz JJ, Simmons AM (1990) Encoding of a spectrally complex natural call in the bullfrog's auditory nerve. J Comp Physiol A 166:489–499
Schwartz JJ, Buchanan BW, Gerhardt HC (2001) Female mate choice in the gray treefrog (Hyla versicolor) in three experimental environments. Behav Ecol Sociobiol 49:443–455
Schwartz J, Buchanan BW, Gerhardt HC (2002) Acoustic interactions among male gray treefrogs, Hyla versicolor, in a chorus setting. Behav Ecol Sociobiol 53:9–19
Schwartz JJ, Huth K, Hutchin T (2004) How long do females really listen? Assessment time for female mate choice in the gray treefrog, Hyla versicolor. Anim Behav 68:533–540
Schwartz JJ, Brown R, Turner S, Dushaj K, Castano M (2008) Interference risk and the function of dynamic shifts in calling in the gray treefrog (Hyla versicolor). J Comp Psychol 122:283–288
Schwartz JJ, Huth K, Hunce R, Lentine B (2010) Effect of anomalous pulse timing on call discrimination by females of the gray treefrog: behavioral correlates of neurobiology. J Exp Biol 213:2066–2072
Schwartz JJ, Crimarco N, Bregman Y, Umeoji K (2013) An investigation of the functional significance of responses of the gray treefrog (Hyla versicolor) to chorus noise. J Herpetol 47:354–360
Slabbekoorn H (2004) Singing in the wild: the ecology of birdsong. In: Marler P, Slabbekoorn H (eds) Nature’s music: the science of birdsong. Academic Press/Elsevier, San Diego, pp 178–205
Sun LX, Wilczynski W, Rand AS, Ryan MJ (2000) Trade-off in short- and long-distance communication in túngara (Physalaemus pustulosus) and cricket (Acris crepitans) frogs. Behav Ecol 11:102–109
Swanson EM, Tekmen SM, Bee MA (2007) Do female frogs use inadvertent social information to locate breeding aggregations? Can J Zool 85:921–932
Taigen TL, Wells KD (1985) Energetics of vocalization by an anuran amphibian (Hyla versicolor). J Comp Physiol B 155:163–170
Vélez A, Bee MA (2011) Dip-listening and the cocktail party problem in grey treefrogs: signal recognition in temporally fluctuating noise. Anim Behav 82:1319–1327
Vélez A, Schwartz JJ, Bee MA (2013) Anuran signal perception in noisy environments. In: Brumm H (ed) Animal Communication and Noise. Springer, New York, pp 133–185
Waser PM, Waser MS (1977) Experimental studies of primate vocalization: specializations for long-distance propagation. Z Tierpsychol 43:239–263
Wells KD (2007) The ecology and behavior of amphibians. University of Chicago Press, Chicago
Wells KD, Schwartz JJ (1982) The effect of vegetation on the propagation of calls in the neotropical frog Centrolenella fleischmanni. Herpetologica 38:449–455
Wells KD, Schwartz JJ (2006) The behavioral ecology of anuran communication. In: Narins PM, Feng AS, Fay RR, Popper AN (eds) Springer handbook of auditory research: hearing and sound communication in amphibians. Springer-Verlag, New York, pp 44–86
Wells KD, Taigen TL (1986) The effect of social interactions on calling energetics in the gray treefrog (Hyla versicolor). Behav Ecol Sociobiol 19:9–18
Wiener FM, Keast DN (1959) Experimental study of the propagation of sound over ground. J Acoust Soc Am 31:724–733
Wilczynski W, Ryan MJ, Brenowitz EA (1989) The display of the blue-black grassquit: the acoustic advantage of getting high. Ethology 80:218–222
Wilczynski W, Rand AS, Ryan MJ (1995) The processing of spectral cues by the call analysis system of the túngara frog, Physalaemus pustulosus. Anim Behav 49:911–929
Wiley RH (2009) Signal transmission in natural environments. In: Squire LR (ed) Encyclopedia of neuroscience volume 8. Academic Press, Oxford, pp 827–832
Wiley RH, Richards DG (1982) Adaptations for acoustic communication in birds: sound propagation and signal detection. In: Kroodsma DE, Miller EH (eds) Acoustic communication in birds, vol 1. Academic Press, New York, pp 131–181
Wollerman L (1999) Acoustic interference limits call detection in a Neotropical frog Hyla ebraccata. Anim Behav 57:529–536
Ziegler L, Arim M, Narins PM (2011) Linking amphibian call structure to the environment: the interplay between phenotypic flexibility and individual attributes. Behav Ecol 22:520–526
Zimmerman BL (1983) A comparison of structural features of calls of open and forest habitat frog species in the central Amazon. Herpetologica 39:235–246
Acknowledgments
We thank Kirsanov Charles, Stephen Sizensky, Reginald Jemison, Stacy Thomas, Jennifer Noviski, and Michael Disimone for help in the lab and field. Jeffrey Storms helped set up the weather station, and Chris Ruthven provided access to the Veteran’s Park during evening hours. We are especially grateful to Kentwood Wells and three anonymous reviewers for valuable comments on the manuscript. This work was supported with Pace University Scholarly Research Awards to JJS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments performed comply with the current laws of the USA and were approved by the Animal Care and Use Committee of Pace University (protocol no. 2009–1).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by K. Summers
Rights and permissions
About this article
Cite this article
Schwartz, J.J., Hunce, R., Lentine, B. et al. Calling site choice and its impact on call degradation and call attractiveness in the gray treefrog, Hyla versicolor . Behav Ecol Sociobiol 70, 1–19 (2016). https://doi.org/10.1007/s00265-015-2016-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-2016-8