Abstract
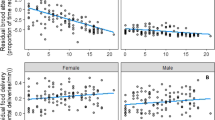
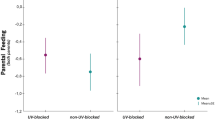
In evolutionary biology, whether parents should enhance or reduce parental care according to mate ornamentation is a subject of great debate. However, the evolution of female ornaments can shed light on this question. In theory, female ornamentation should be traded off against fecundity and thus cannot be wholly informative to males without a direct indication of fecundity. Hence, direct cues of offspring quality should affect the relationship between male investment and female ornamentation. Under this hypothesis, we manipulated two direct cues of offspring quality (egg size and color) after first egg laying in the blue-footed booby and registered male incubation patterns. In this species, foot color is a dynamic signal of current condition and in females is traded off with egg size. We found that males spent more time incubating when paired with dull females but only in nests with large eggs. Males also spent less time incubating small dull eggs. Results indicate that egg size, a direct cue of reproductive value, affected the relationship between male effort and female ornamentation. Males may be willing to help females that have invested in offspring at the expense of ornamentation, which suggests compensation when females are in low condition. Another possibility is that males relax their effort when paired with highly ornamented and fecund females because they have high parenting abilities. Our findings suggest that the information conveyed by female ornaments may depend on direct cues of fecundity. Results also highlight that parental decisions are complex, modulated by a combination of information sources.



Similar content being viewed by others
References
Ancona S, Sánchez-Colón S, Rodríguez C, Drummond H (2011) El niño in the warm tropics: local sea temperature predicts breeding parameters and growth of blue-footed boobies. J Anim Ecol 80:799–808
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Barta Z, Houston AI, McNamara JM, Székely T (2002) Sexual conflict about parental care: the role of reverses. Am Nat 159:687–705
Bolund E, Schielzeth H, Forstmeier W (2009) Compensatory investment in zebra finches: females lay larger eggs when paired to sexually unattractive males. Proc R Soc Lond B 276:707–715
Braga-Goncalves I, Mobley KB, Ahnesjö I, Sagebakken G, Jones AG, Kvarnemo C (2010) Reproductive compensation in broad-nose pipefish females. Proc R Soc Lond B 277:1581–1587
Burley N (1986) Sexual selection for aesthetic traits in species with biparental care. Am Nat 127:415–445
Burley N (1988) The differential allocation hypothesis: an experimental test. Am Nat 132:611–628
Byers JA, Waits L (2006) Good genes sexual selection in nature. Proc Natl Acad Sci U S A 103:16343–16345
Chenoweth SF, Doughty P, Kokko H (2006) Can non-directional male mating preferences facilitate honest female ornamentation? Ecol Lett 9:179–184
Cunningham EJA, Russell AF (2000) Egg investment is influenced by male attractiveness in the mallard. Nature 404:74–77
D'Alba L, Torres R (2007) Seasonal egg-mass variation and laying sequence in a bird with facultative brood reduction. Auk 124:643–652
Davis JN, Todd PM, Bullock S (1999) Environmental quality predicts parental provisioning decisions. Proc R Soc Lond B 266:1791–1797
Dentressangle F, Boeck L, Torres R (2008) Maternal investment in eggs is affected by male feet colour and breeding conditions in the blue-footed booby, Sula nebouxii. Behav Ecol Sociobiol 62:1899–1908
Fitzpatrick S, Berglund A, Rosenqvist G (1995) Ornaments or offspring: costs to reproductive success restrict sexual selection processes. Biol J Linn Soc 55:251–260
Folstad I, Carter AJ (1992) Parasites, bright males, and the immunocompetence handicap. Am Nat 139:603–622
Fox J (1997) Applied regression analysis, linear models, and related methods. Sage, Thousand Oaks
García-Peña GE (2005) Efectos y costos de la termorregulación durante la incubación del ave marina Sula nebouxii. Mater thesis, Universidad Autónoma Nacional de México, México
Gowaty PA (2008) Reproductive compensation. J Evol Biol 21:1189–1200
Gowaty PA, Anderson WW, Bluhm CK, Drickamer LC, Kim YK, Moore AJ (2007) The hypothesis of reproductive compensation and its assumptions about mate preferences and offspring viability. Proc Natl Acad Sci U S A 104:15023–15027
Harris WE, Uller T (2009) Reproductive investment when mate quality varies: differential allocation versus reproductive compensation. Phil Trans R Soc B 364:1039–1048
Hill JA, Enstrom DA, Ketterson ED, Nolan V Jr, Ziegenfus C (1999) Mate choice based on static versus dynamic secondary sexual traits in the dark-eyed junco. Behav Ecol 10:1–96
Hinde CA, Kilner RM (2007) Negotiations within the family over the supply of parental care. Proc R Soc Lond B 274:53–60
Hoelzer GA (1989) The good parent process of sexual selection. Anim Behav 38:1067–1078
Krebs EA, Hunte W, Green D (2004) Plume variation, breeding performance and extra-pair copulations in the cattle egret. Behaviour 141:479–499
Lessells CM (1999) Sexual conflict in animals. In: Keller L (ed) Levels of selection in evolution. Princeton University Press, Princeton, pp 75–99
Matessi G, Carmagnani C, Griggio M, Pilastro A (2009) Male rock sparrows differentially allocate nest defence but not food provisioning to offspring. Behaviour 146:209–223
Morales J, Alonso-Álvarez C, Pérez C, Torres R, Serafino E, Velando A (2009a) Families on the spot: sexual signals influence parent–offspring negotiations. Proc R Soc Lond B 276:2477–2483
Morales J, Velando A, Torres R (2009b) Fecundity compromises attractiveness when pigments are scarce. Behav Ecol 20:117–123
Morales J, Velando A, Torres R (2010a) Biliverdin-based egg coloration is enhanced by carotenoid supplementation. Behav Ecol Sociobiol 65:197–203
Morales J, Torres R, Velando A (2010b) Parental conflict and blue egg coloration in a seabird. Naturwissenschaften 97:173–180
Moreno J, Osorno JL (2003) Avian egg color and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecol Lett 6:803–806
Nelson JB (1978) The Sulidae: gannets and boobies. Oxford University Press, Oxford
Osorno JL, Székely T (2004) Sexual conflict and parental care in magnificent frigatebirds: full compensation by deserted females. Anim Behav 68:337–342
Pérez-Staples D, Drummond H (2005) Tactics, effectiveness and avoidance of mate guarding in the blue-footed booby. Behav Ecol Sociobiol 59:115–119
Pilastro A, Griggio M, Matessi G (2003) Male rock sparrows adjust their breeding strategy according to female ornamentation: parental or mating investment? Anim Behav 66:265–271
Ratikainen II, Kokko H (2010) Differential allocation and compensation: who deserves the silver spoon? Behav Ecol 21:195–200
Reed JR (1987) Scotopic and photopic spectral sensitivities of boobies. Ethology 76:33–55
Reznick D, Nunney L, Tessier A (2000) Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol Evol 15:421–425
Roff DA, Fairbairn DJ (2007) The evolution of trade-offs: where are we? J Evol Biol 20:433–447
Roulin A (1999) Non-random pairing by male barn owls (Tyto alba) with respect to a female plumage trait. Behav Ecol 10:688–695
Roulin A, Altwegg R, Jensen H, Steinsland I, Schaub M (2010) Sex-dependent selection on an autosomal melanic female ornament promotes the evolution of sex ratio bias. Ecol Lett 13:616–626
Saino N, Bertacche V, Ferrari RP, Martinelli R, Møller AP, Stradi R (2002) Carotenoid concentration in barn swallow eggs is influenced by laying order, maternal infection and paternal ornamentation. Proc R Soc Lond B 269:1729–1733
Sheldon B (2000) Differential allocation: tests, mechanisms and implications. Trends Ecol Evol 15:398–402
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stocker R, Yorihiro Y, McDonagh AF, Glazer AN, Ames BN (1987) Bilirubin is an antioxidant of possible physiological properties. Science 235:1043–1046
Torres R, Drummond H (1999) Does large size make daughters of the blue-footed booby more expensive than sons? J Anim Ecol 68:1133–1141
Torres R, Velando A (2003) A dynamic trait affects continuous pair assessment in the blue-footed booby (Sula nebouxii). Behav Ecol Sociobiol 55:65–72
Torres R, Velando A (2005) Male preference for female foot color in the socially monogamous blue-footed booby Sula nebouxii. Anim Behav 69:59–65
Torres R, Velando A (2010) Color in a long-lived tropical seabird: sexual selection in a life-history context. Advances 42:155–188
Trivers RL (1972) Parental investment and sexual selection 1871–1971. In: Campbell BG (ed) Sexual selection and the descent of man. Aldine, Chicago, pp 136–179
Velando A, Torres R, Espinosa I (2005) Male coloration and chick condition in blue-footed booby: a cross-fostering experiment. Behav Ecol Sociobiol 58:175–180
Velando A, Beamonte-Barrientos R, Torres R (2006) Pigment-based skin colour in the blue-footed booby: an honest signal of current condition used by females to adjust reproductive investment. Oecologia 149:535–542
Wiebe KL (2010) Negotiation of parental care when the stakes are high: experimental handicapping of one parent during incubation leads to short-term generosity. J Anim Ecol 79:63–70
Williams GC (1966) Natural selection, the costs of reproduction and a refinement of Lack's principle. Am Nat 100:687–690
Winkler DW (1987) A general model of parental care. Am Nat 130:526–543
Winkler DW, Wallin K (1987) Offspring size and number: a life history model linking effort per offspring and total effort. Am Nat 129:709–720
Zavalaga CB, Benvenuti S, Dall'Antonia L, Emslie SD (2007) Diving behavior of blue-footed boobies Sula nebouxii in northern Peru in relation to sex body size and prey type. Mar Ecol Prog Ser 336:291–303
Acknowledgments
We are grateful to four anonymous reviewers for valuable and constructive comments. We thank René Beamonte-Barrientos, Natalia Lifshitz, and Alejandro Mendez for help in the field. We thank the fisherman from San Blas and Camichín, the staff from Parque Nacional Isla Isabel, and the Armada de México for logistic support. SEMARNAT gave working permissions and approved the research. This work was supported by the Universidad Nacional Autónoma de México (PAPIIT IN211406, IN228309), Consejo Nacional de Ciencia y Tecnología (47599, 81823) of México, Ministerio de Ciencia e Innovación (CGL2006-10357-C02-01/CGL2009-10883-C02-01 and Juan de la Cierva fellowship to JM) of Spain, and a research grant (2007) from the Association for the Study of Animal Behaviour to JM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Pruett-Jones
Rights and permissions
About this article
Cite this article
Morales, J., Torres, R. & Velando, A. Safe betting: males help dull females only when they raise high-quality offspring. Behav Ecol Sociobiol 66, 135–143 (2012). https://doi.org/10.1007/s00265-011-1261-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1261-8