Abstract
An individual’s decision to disperse from the natal habitat can affect its future fitness prospects. Especially in species with sex-biased dispersal, we expect the cost–benefit balance for dispersal to vary according to the social environment (e.g., local sex ratio and density). However, little is known about the social factors affecting dispersal decisions and about the temporal and spatial patterns of the dispersal process. In our study, we investigated experimentally the effects of the social environment on post-fledging dispersal of juvenile great tits by simultaneously manipulating the density and sex ratio of fledglings within forest plots. We expected young females in the post-fledging period mainly to compete for resources related to food and, as they are subordinate to males, we predicted higher female dispersal from male-biased plots. Juvenile males compete for vacant territories already in late summer and autumn; thus, we predicted increased male dispersal from high density and male-biased plots. We found that juvenile females had a higher probability to leave male-biased plots and had dispersed further from male-biased plots in the later post-fledging phase when juvenile males start to become territorial and more aggressive. Juvenile males were least likely to leave male-biased plots and had smallest dispersal distances from female-biased plots early after fledging. The results suggest that the social environment differentially affected the costs and benefits of philopatry for male and female juveniles. The local sex ratio of individuals is thus an important social trait to be considered for understanding sex-specific dispersal processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In most species of mammals and birds, males and females differ in the extent of natal dispersal (Greenwood 1980; Dobson 1982; Greenwood and Harvey 1982; Pusey 1987). Theoretical models have identified inbreeding avoidance (Perrin and Mazalov 2000; Gros et al. 2008) and also sex-specific costs and benefits of dispersal as potential ultimate causes for sex-biased dispersal (Perrin and Mazalov 2000; Wild and Taylor 2004; Gros et al. 2008). Greenwood (1980) specified that the type of breeding system (resource defense or mate defense mating system) should create asymmetries between the sexes in the extent of intra-sexual competition for resources and thus also create sex-specific differences in the relative costs and benefits of dispersal.
In birds, females are generally the more dispersive sex (Greenwood and Harvey 1982; Clarke et al. 1997). An explanation for this may be that, in most bird species, males defend breeding territories (Greenwood 1980; Pusey 1987). Accordingly, males should have higher chances of establishing and keeping a breeding territory if they have prior knowledge of the breeding habitat. Females, on the other hand, may benefit from dispersing by being able to choose among potential territory holders. Thus, the benefits of staying close to the natal territory should be higher for males. However, the numbers and sex ratio of conspecifics in an area will change the sex-specific competition for resources and thus directly affect the probability for a male to gain a territory and for a female to find a suitable partner. Additionally, outside the breeding period, the larger sex often dominates the smaller one in competitive interactions over resources such as food (Peters and Grubb 1983; Hogstad 1989; Tarvin and Woolfenden 1997; Marra 2000) or roosting sites (Kluyver 1957; Summers et al. 1986; Feare et al. 1995). In such a context, the local density and sex ratio of conspecifics are expected to affect the cost–benefit balance of dispersal. Juvenile birds’ decisions on whether and how far to disperse should thus depend on the extent to which the social environment is repulsive or attractive. There exist a number of studies on density effects on dispersal in mammals and birds, mostly showing positive density dependence of dispersal distances or dispersal rates (see Matthysen 2005 for a review). Studies on sex-specific density effects on dispersal are rather scarce until now, especially in birds, and suggest that population density and sex ratio primarily influence the level of competition for mates (Wilson and Arcese 2008; Richardson et al. 2010).
The dispersal process consists of several phases, starting with the decision to leave an area, followed by a transient phase when a new habitat is searched for, and ending with final settlement (Bennetts et al. 2001). Competition may play a role in any of these stages, but selection pressures may differ in each phase. Investigating only effective dispersal (movement that is followed by reproduction; Greenwood 1980) can lead to misinterpretations of the adaptiveness of dispersal decisions because only the decisions of birds that survived the dispersal process are taken into account. Furthermore, the fate of long-distance dispersers leaving a study area is often not known (Barrowclough 1978; Greenwood and Harvey 1982). Studying the spatial and temporal patterns of movement in the early phase after young birds become independent should provide more insight into the proximate causes of dispersal.
The great tit (Parus major) system is ideal for the study of the importance of local density and sex ratio for decisions involved in dispersal behavior. Male great tits are territorial and young males can already start to establish a breeding territory in autumn (Kluyver 1951; Drent 1983). Female great tits are consistently the sex with larger natal dispersal distances (Greenwood et al. 1979; Verhulst et al. 1997; Dingemanse et al. 2003; Tinbergen 2005; Matthysen et al. 2010), and females already move larger distances from the natal habitat shortly after the breeding season (Dhondt 1979; Drent 1984). Furthermore, female great tits are generally subordinate to males in competition for artificial food (Kluyver 1957; Drent 1983; Wilson 1992), and females probably also lose from males in competition for roosting sites in winter (Kluyver 1957). These characteristics offer the possibility that local changes in density and sex ratio affect the levels of competition between and within the sexes. Earlier non-experimental studies in great tits that investigated sex-specific dispersal patterns in relation to local competition found variable results. Greenwood et al. (1979) showed that, when the number of breeding pairs in the population was high, females moved smaller distances while males moved more territories. Delestrade et al. (1996) found that male and female dispersal distances did not vary with local densities, but males which changed habitat settled in areas with low occupation rates. Drent (1984) concluded from his post-fledging study in two Dutch populations that dispersal rate was independent of density but varied between areas. Because density is often positively related to resource availability, natural densities may not correlate with the levels of resource competition. Therefore, densities should be manipulated to disentangle competition-related effects from the effects of local habitat quality.
In our study, we investigated experimentally whether and how sex-specific dispersal in a wild great tit population was affected by the levels of local competition. For this purpose, we manipulated the local social environment (density and sex ratio) of juvenile great tits on the level of forest plots in 2 years. In each year, we monitored post-fledging movements of juvenile birds until October to investigate the aspects of spatial and temporal sex-specific dispersal patterns. If competition occurs mainly within the sexes in the period studied (e.g., competition for partners or territories), then we expect males to disperse further from male-biased plots and females from female-biased plots. If competition acts mostly between the sexes, we expect females to suffer from a male-biased local sex ratio and thus to disperse further from such plots. Both sexes should suffer from increased competition if local densities are high in the post-fledging period and thus disperse further, although male territorial behavior might render males more sensitive to changes in local density. We investigate dispersal as (a) the dispersal distance in a model using all sightings of individuals and the days observed after fledging as covariate to explore the temporal course of events. This dependent variable investigates how far individuals move after a certain experimental treatment. Furthermore, we investigate (b) the probability that a bird left the plot at least once during the period studied. This analysis should help to distinguish between-plot movements from within-plot movements. Our study will provide a more detailed insight in the processes involved in sex-specific dispersal as well as additional knowledge on the causal factors that influence an individual’s decision to disperse.
Methods
Study area
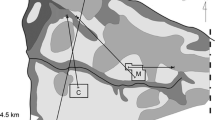
The study was conducted in the Lauwersmeer area which is situated in the northeast of The Netherlands (53°23′ N, 6°14′ E). In February 2005, we established 12 nest box plots, where in some woodlots existing boxes were already rearranged and in others they were newly put up (Fig. 1). Each plot consisted of 50 nest boxes in a regular 50-m grid. The woodlots were primarily deciduous forests (about 30-year-old plantations of mainly oak (Quercus robur), poplar species (Populus sp.), birch (Betula pubescens), maple (Acer platanoides), ash (Fraxinus excelsior), and elder (Alnus glutinosa)) and were separated by at least 300 m of open grassland or forest patches without boxes.
Field and molecular methods
From the beginning of April, we checked the nest boxes weekly to establish the start of egg laying (if necessary, we back-calculated by assuming that one egg was laid per day) and we determined the clutch size after the onset of incubation. Before the expected hatching date, we checked the nest boxes daily to determine the hatching date of the first eggs in the nest (day 0). We sampled a small quantity of blood (ca. 5–10 μl) from the tarsal vein of all nestlings when they were 2 days old and clipped the end of their toenails in a unique combination for later identification of individuals (St. Louis et al. 1989). Between days 3 and 5, we performed molecular sex determination such that on day 6, after hatching, we knew the sex of all nestlings to allow accurate sex ratio manipulation of the broods (see below). We extracted DNA using the Chelex method described by Walsh et al. (1991). Sex of the young was determined following Griffiths et al. (1998). The PCR products were separated by electrophoresis on 2% agarose gel.
On day 6, the nestlings received a numbered aluminum ring and we exchanged them between broods of the same age to achieve nest and plot level manipulations (see the following paragraphs). At day 14, after hatching, we provided all nestlings additionally with three color rings (11 different colors were used) in a unique combination with the aluminum ring. Nestlings leave the nest approximately 20 days after hatching; thus, we performed nest box checks every second day from day 19 onward to determine successful fledging of nestlings.
Experimental design
We simultaneously manipulated plot density (number of nestlings) and plot sex ratio (proportion of males) of juvenile great tits in 2005 and 2006 by manipulating brood sex ratios and brood sizes within plots. Plot sex ratio treatments were either male-biased (75% male young), female-biased (25% male young), or control (50% male young), and the plot density treatments were high (increased number of young) or low density (reduced number of young; Fig. 2). We created six different combinations of sex ratio and density treatments ranging from male-biased/low density to female-biased/high density plots (Fig. 2). Each treatment combination occurred in two replicates per year and was semi-randomly allocated to plots each year (such that no plot had the same combination in consecutive years). This randomization of plot treatments should reduce the influence of plot effects (plot quality, distance to other plots, or degree of isolation) in our data. To achieve the plot treatments, we manipulated first broods (broods that started less than 30 days after the start of the very first brood in that year) within plots at day 6 after hatching to receive the treatment that corresponded to the plot treatment (Fig. 2). For the sex ratio, this meant that we manipulated all broods within a plot in the direction of the plot treatment (Fig. 2). Thus, we manipulated broods to contain on average about 25% male young in female-biased plots, 50% male young in control plots, and 75% male young in male-biased plots (Fig. 2; “Electronic supplementary material”, Fig. 1). Variation in experimental brood sex ratios within plots occurred because the brood sizes varied and because occasionally there were not enough or too many individuals of a given sex available for broods with the same hatching date. The density manipulation was done by manipulating 60% of the broods within plots in the desired direction (Fig. 2; “Electronic supplementary material”, Fig. 2). So, to achieve a high density treatment, we increased the brood size for the majority of broods in this plot and we kept 20% of broods per plot as opposite treatment controls (reduced brood size for high density plots) and 20% as real controls (average brood size for a year). These controls were used to study the interaction between density and brood size on breeding output as presented in Nicolaus et al. (2009). We randomly assigned brood size treatments to nests within plots before clutch completion. Control broods were manipulated to contain the “average” brood size for a given year; thus, because of yearly variations in average brood size in our population, experimental control brood size centered around seven or eight nestlings in 2005 and nine nestlings in 2006. Broods that were assigned the “enlarged” treatment were manipulated to contain a brood size of on “average”+3 nestlings (10–12 depending on the year) and broods in the reduced treatment received a brood size of “average” −3 nestlings (four to six depending on year; “Electronic supplementary material”, Fig. 2). We give averages per year for the plot treatment categories in Table 1. We only manipulated first broods, second broods and replacement broods of known first broods after failures were left unmanipulated.
Experimental treatments applied to plots during the 2 years, 2005 and 2006, combining sex ratio and density of juvenile great tits. The sex ratio treatment at the plot level was achieved by manipulating all broods within the plot in the same direction, while for the density treatment we manipulated 60% of the broods within a plot towards the desired treatment, keeping 40% of the nests as controls for the other treatments. F female-biased brood, C control sex ratio brood, M male-biased brood, R reduced brood size, C control brood size, E enlarged brood size
The three plot sex ratio treatment categories did not differ in natural (premanipulation) nestling sex ratio at day 6 (Kruskal–Wallis test: χ 2 = 1.00, df = 2, p = 0.606) and the two density treatment categories also did not differ in natural (premanipulation) density of nestlings (independent t-test: t = 0.399, df = 22, p = 0.694).
The plot sex ratio treatment has been very successful in changing the proportion of male nestlings between plots while keeping some variation in brood sex ratios within plots (“Electronic supplementary material”, Fig. 1). The three plot sex ratio categories thus differed significantly in final nestling sex ratio at day 6 (female-biased = 0.24 ± 0.01 SE, control = 0.49 ± 0.01 SE, male-biased = 0.75 ± 0.01 SE; Kruskal–Wallis test: χ 2 = 16.00, df = 2, p < 0.001). The natural plot sex ratio and the final experimental plot sex ratio did therefore not correlate after manipulation (Spearman rank correlation, r s = 0.230, n = 24, p = 0.280).
The density treatment resulted on average in a density change of ±17 young per plot that significantly differed between the treatment groups (independent t-test: t = −8.21, df = 22, p < 0.001). The density treatment categories differed significantly in final nestling density at day 6 in 2005 but not in 2006 (2005: independent t-test: t = −3.366, df = 10, p = 0.007; 2006: t = −0.541, df = 10, p = 0.600) because in 2006 the average natural density was by chance already slightly higher in low density plots than in high density plots (Table 1). The final experimental density of nestlings per plot was thus still strongly correlated to the natural density of nestlings per plot (Pearson correlation, r c = 0.860, n = 24, p < 0.001) which was mainly because not all broods were biased in the direction of the plot treatment (Fig. 2).
Observations
One week after the earliest first broods had fledged in 2005 and 2006, we started observations of color-ringed great tits to cover most of the forested part of the study area (Fig. 1). Fledging of the very first brood in 2005 occurred on the 25th of May (± 1 day) and in 2006 on the 29th of May (± 1 day). The last first broods fledged around 27th of June (± 1 day) in 2005 and around the 19th of June (± 1 day) in 2006. The forested area (dark gray area in Fig. 1) was divided in 12 parts (numbered observation plots in Fig. 1) of about 30–50 ha each, and in every part we spent 4 h per observation occasion (observation day for a part/mostly 2 days for the whole area) to search for great tits. Individuals were located by sound and sight, and we tried to read their color rings with binoculars (10 × 40, 10 × 42). We wrote down the location for each group or individual where they were first seen and noted down all read color rings. For each single color ring, we also noted down whether we considered the color detection certain (certainty was often based on repeated readings). Observations followed a regular schedule with at least biweekly observation occasions in June, July, August, September, and October. To reduce observer effects, the observers switched plots between occasions.
Data selection and analyses
For data analyses on dispersal behavior, we used sightings of all first brood juvenile great tits that were known to have fledged in 2005 and 2006. We only included readings where the color detection had been certain for all rings in the combination. We used observations from the fourth week after fledging until the end of the observation period in October (max. 148 days post-fledging). The first 4 weeks after fledging were omitted from the analysis because in great tits post-fledging care continues on average for about 20 days (Drent 1984; Verhulst and Hut 1996) but can continue up to 30 days (Verhulst and Hut 1996). In this early post-fledging phase, movements of juveniles are thus likely to be influenced by their parents and effects of the treatment on juvenile movement could also result from parental behavior.
In a first analysis, we investigated “dispersal distance” to see how far individuals move after a certain experimental treatment. This is informative because a plot’s treatment is likely to expand beyond plot borders in the course of time due to the movement of birds, and competition is then no longer avoided by leaving a plot (analysis is explained below). In the model, we used one sighting per individual for each observation day as dependent variable and the time individuals were observed after fledging as covariate (days since fledging). This was done because (a) birds are more likely to be observed further from the nest box of fledging the longer since they have fledged; (b) The type of resources juveniles compete for are likely to change during the period of interest. For example, food is likely to be the most important resource juveniles compete for in summer but sex-specific behaviors such as territorial defense become more important in autumn and therefore also the scope for competition between males; (c) Due to movements of birds, the experimental plot compositions will change and the bias in plot density and plot sex ratio will decrease with increasing time since fledging (“Electronic supplementary material”, Figs. 3 and 4). Therefore, we need to investigate whether sex-specific responses to experimental changes in plot density and plot sex ratio vary with increasing time since fledging (interaction: sex × treatment × days since fledging). The interaction between “days since fledging” and the other variables of interest (sex, natural density, plot sex ratio, and density treatment) are interpreted as “timing of dispersal” effect (at what time individuals are observed far from the nest box of origin), e.g., a significant interaction of sex × “days since fledging” for dispersal distance would indicate a sex difference in “timing of dispersal”. For the analysis of “dispersal distance”, we used the sighting per day with the maximum distance seen from the nest box of origin because birds were sporadically seen more often during an observation day. Therefore, the term “sighting” used in the analyses is equivalent to “observation day”. This gave a sample size of 1,906 sightings for 1,086 individual juvenile great tits.
In a second analysis, we examined the probability that a bird left the plot at least once during the period studied. In this analysis, the temporal pattern of dispersal is disregarded, but we can simply test whether the plot treatment affected the birds to leave a certain plot. For this analysis, we assigned to each sighting of an individual whether it was in or outside the plot it had fledged from (accurate to about ±25 m). The bird was then assigned to whether it was observed during the study period at least once outside the plot or not using a binary variable (n = 1,086 individuals).
Our data set had a strong hierarchical structure with individual sightings (maximal dispersal distance within a day) nested within individuals, within broods, cohorts (all broods within a plot in a given year), and plots. Therefore, we used linear mixed models (MLwiN 2.0; Rasbash et al. 2004) to account for interdependencies of records within a level. In the first analysis, plot, cohort, brood, individual, and sighting were thus included as random effects and in the second analysis plot, cohort, brood, and individual. The dependent variable in the first analysis was the distance of observation from the nest box of fledging and was transformed by taking the log10 to allow analyses using normal response models. In the second analysis, the probability that a bird has left the plot at least once was analyzed in a binomial response model with a logit-link function (first-order PQL estimation procedure).
We analyzed dispersal distance in relation to the categorical predictors sex (female as reference), plot sex ratio (three categories, control as reference), and density treatment (low as reference) and the continuous predictors natural plot density and days since fledging (number of days from fledging until the observation, accuracy of ± 1 day, all centered on their overall averages over both years). The effects of the plot sex ratio and density treatment were analyzed to detect causal relationships between the social environment and dispersal. We analyzed the effect of the natural density of nestlings per plot as non-experimental density variable that might relate to the availability of resources such as food, roosts, or territories. To investigate the sex-specific effects of the experimental treatment and natural density on dispersal, we tested two-way interactions between sex and the plot sex ratio treatment and density treatment and natural density, respectively. To look at the effects of the manipulated social environment (combined plot density and plot sex ratio effects), we also tested two-way interactions between the plot sex ratio treatment and density treatment/natural density and three-way interactions between sex, the plot sex ratio treatment, and the density treatment or natural density, respectively, but as they were never significant we do not report those results. Because the density treatment categories differed in final nestling densities in 2005 but not in 2006 (see above text), we tested whether the effect of the density treatment on dispersal distance and the probability to leave a plot differed between the 2 years. This was not the case (keeping all underlying effect in the model for dispersal distance: year × density treatment: χ 2 = 0.282, df = 1, p = 0.595; for probability to leave: χ 2 = 0.003, df = 1, p = 0.956). We also tested interactions with days since fledging (timing of dispersal) as described above. For the probability to leave a plot, we tested the same variables as for dispersal distance except the covariate days since fledging and the interactions with it (because only one record per individual was used).
In addition to the plot variables, we tested whether our results were not caused by competition in the nestling phase, thus whether the respective brood manipulation at the nest level would change the results for plot treatment effects when included in the model. Thus, we included experimental brood size categories (reduced, control, and enlarged, with control as reference) in the model if density treatment would be significant. Because we manipulated plot sex ratio by manipulating the sex ratio of all broods in a plot in the same direction, it is impossible to fully disentangled plot sex ratio from brood sex ratio effects using treatment categories. Therefore, in those cases where we found an effect of the plot sex ratio treatment, we also analyzed the effect of brood sex ratio manipulation by including the deviance of the final experimental brood sex ratio from the final experimental plot sex ratio (referred to as the relative experimental brood sex ratio) while keeping the plot sex ratio treatment in the model. Those results are only reported when applicable.
We applied Wald test to determine the significance of explanatory variables as they were removed from the model. It occurred that variation for some of the specified random effects could not be estimated. This indicates that there was too little variation in the dependent variable on these levels or that the underlying levels took over all the variations. Excluding levels with zero estimates from the analyses did not change the results, so we always kept them in the models.
Results
Juvenile great tits in the post-fledging period moved away from the nest box of fledging up to an average distance of 1,307.58 m ± 23.25 SE. Juvenile females moved on average further from the nest box of fledging than juvenile males (females, 1,376.70 m ± 33.24 SE; males, 1,241.90 m ± 32.416 SE; χ 2 = 5.700, df = 1, p = 0.017; tested in a final model where any interaction with sex was removed).
Effect of plot treatments on dispersal distance
We found that the experimental plot sex ratio affected the juvenile timing of dispersal differently for males and females (interaction sex × plot sex ratio treatment × days since fledging; Table 2; Fig. 3). Juvenile females from male-biased plots increased their distance from the nest box of fledging more strongly with days since fledging than females from control and female-biased plots (Table 2; Fig. 3). Juvenile males from control and male-biased plots were observed at about an equal distance from the nest box of fledging over the entire post-fledging period while juvenile males from female-biased plots, on the other hand, were observed closer in the beginning of the post-fledging period but farther away later on. This effect remained significant (sex × plot sex ratio treatment × days: χ 2 = 10.950, df = 2, p = 0.004) if we controlled the model for the same interaction on the brood level (sex × relative experimental brood sex ratio × days: χ 2 = 0.586, df = 1, p = 0.444). The density treatment did not affect the dispersal distance (β = −0.072 ± 0.071 SE, χ 2 = 1.018, df = 1, p = 0.313) nor the timing of dispersal (density treatment × days: χ 2 = 0.031, df = 1, p = 0.860) and did not affect the dispersal distance or timing of dispersal differently for males and females (sex × density treatment: χ 2 = 0.169, df = 1, p = 0.681, sex × density treatment × days: χ 2 = 0.011, df = 1, p = 0.916).
Predicted distances moved (on a log scale) with days since fledging of juvenile female (left graph) and male (right graph) great tits in the entire post-fledging period in relation to the plot sex ratio treatment (dotted lines for female-biased plots, dashed lines for control plots, and solid lines for male-biased plots) for the years 2005 and 2006. Lines are based on model predictions of the interaction sex × sex ratio treatment × days since fledging in the final model
The two offspring sexes did not differ in how the natural density in the plot of fledging related to the distances moved in the post-fledging phase (sex × natural density: χ 2 = 2.390, df = 1, p = 0.122) or to the timing of dispersal (sex × natural density × days: χ 2 = 0.021, df = 1, p = 0.885).
The dispersal distance was significantly affected by the interaction between the natural density of young in a plot and days since fledging (Table 2; Fig. 4). Juveniles that fledged from high natural density plots had moved larger distances than juveniles from plots with low natural densities in the beginning of the post-fledging period, but later on the distances were more similar (Table 2; Fig. 4). This pattern did not differ between the sexes (all other terms included in the model, sex × natural density × days since fledging: χ 2 = 0.021, df = 1, p = 0.885).
Predicted distances moved (on a log scale) with days since fledging of juvenile great tits in the post-fledging period in relation to the natural plot density of nestlings for the years 2005 and 2006. For graphical representation, the natural plot density was assembled in plots with lower (75–120, dotted line), average (121–165, dashed line), and higher than the average natural plot density of nestlings (165–210, solid line) over the 2 study years (total average density, 142.83 ± 35.14 SD). Lines are based on model predictions of the interaction natural density × days since fledging in the final model
Effect of plot treatments on probability to leave a plot
We found that male and female juveniles differed in how the experimental plot sex ratio affected the probability that a juvenile left the plot at least once during the study period (interaction sex × plot sex ratio treatment; Table 3; Fig. 5). Male and female juveniles were equally likely to leave female-biased plots during the post-fledging period but differed in the probability to leave control and male-biased plots (Fig. 5). Female juveniles were more likely to leave a male-biased plot than male juveniles but less likely to leave a control plot.
The density treatment (β = −0.115 ± 0.403 SE, χ 2 = 0.081, df = 1, p = 0.776) and the natural density of nestlings (β = 0.07 ± 0.006 SE, χ 2 = 1.293, df = 1, p = 0.255) were not related to the probability that a juvenile left the plot of fledging and the sexes also did not differ in their response to the density treatment (sex × density treatment: β = 0.351 ± 0.521 SE, χ 2 = 0.454, df = 1, p = 0.500) or the natural density of nestlings in the plot of fledging (sex × natural density: β = 0.013 ± 0.008 SE, χ 2 = 2.476, df = 1, p = 0.115).
Discussion
We expected that the local social environment would affect the dispersal decisions of juvenile great tits after fledging. Depending on the period but also the juvenile’s sex, a certain social setting may be more or less attractive. We predicted that, if competition mainly takes place within the sexes, females should disperse further from female-biased plots and males from male-biased plots. If competition between the sexes is more important, we expected female young to be outcompeted by male young and thus to disperse further from male-biased plots. Our results showed that, for juvenile females, competition with males seemed most important during the post-fledging period as females had dispersed further from male-biased plots than from control or female-biased plots at the end of the post-fledging phase (Fig. 3). Juvenile males generally disperse less far than females but showed a different timing of dispersal in female-biased plots. These sex-specific experimental effects on dispersal distance were not likely caused by only certain individuals surviving or being observed in the period studied (survival and re-sighting probability of juveniles did not depend sex-specifically on the plot sex ratio treatment (Michler 2010)). Male and female juveniles’ probability to leave the plot of fledging was also differently affected by the plot sex ratio treatment and is partly consistent with the patterns found for dispersal distance (Fig. 5). Female juveniles had a higher probability to leave male-biased plots than male juveniles, but males were more likely to leave control plots than females. Differences between the dispersal distance analysis and the probability to leave a plot analysis might originate because the more complex (less simplified) dispersal distance analysis additionally covers the temporal aspect of the dispersal process and also from the different spatial scales that are covered by the two. Smaller dispersal distances do not necessarily indicate that a juvenile stayed in the plot of fledging. On the other hand, juveniles that left a plot might have been observed “just” outside a plot and thus not necessarily find themselves in a different social setting because the movement of juveniles was directed away from the nest box of fledging and the social setting most likely expanded beyond plot borders. We will incorporate the findings for both the dispersal distance and the probability to leave a plot when discussing the differences between juvenile males and females in their response to the local social environment, and we will examine the temporal patterns of their dispersal decisions.
Dispersal process
Our data showed that juvenile great tits of both sexes increased their distances from the nest box of fledging strongly during the first month post-fledging (unpublished data) and continued to move farther afterwards, which was indicated by an increase in dispersal distance with increasing time since fledging (Table 2).
The dispersal process in great tits is composed of several stages. In an initial period of about 10–20 days, the juveniles are still dependent on their parents for receiving food (Hinde 1952; Drent 1984; Verhulst and Hut 1996) and finding suitable feedings sites (Drent 1984). Later, the juveniles become independent and build up flocks that vary in size and composition of individuals from different sexes, age classes, and even species (Hinde 1952; Hogstad 1989). The juveniles first move around in summer flocks that later break up to build more stable basic flocks in late September (Saitou 1979a). Most young settle in a restricted area from September onward (Saitou 1979a), but some might still wander around during winter (Hinde 1952). Our results support these previous findings because juvenile movement away from the nest box of fledging did not stop after the juveniles gained independence but continued at least until October.
Overall, juvenile females were observed at larger distances from the nest box of origin than juvenile males, an observation which is in agreement with the findings of other great tit studies (Dhondt 1979; Drent 1984). This suggests that important selection pressures responsible for the sex difference in natal dispersal distance in this species already act early after the birds leave the nest.
Sex-specific experimental effects of plot sex ratio
We showed that, later in the post-fledging period, juvenile females had moved larger distances from male-biased plots than juvenile males. Additionally, juvenile females were more likely to be observed outside male-biased plots than juvenile males. This behavior of juvenile females might have allowed them to avoid male-biased plots later in the season, which is about the period of territory establishment in September and October (days 100–140). In spite of the high levels of movement in the post-fledging phase, the plots still showed a sex ratio bias in October, at least in some years (“Electronic supplementary material”, Fig. 3). Therefore, our results suggest that females avoided competition for male-dominated resources such as food or potentially roosting sites. Competition for these resources is likely to increase in autumn (late September, October) when nights become colder and food is more restricted. The increased dispersal of females from male-biased plots might thus be a response to high levels of aggressiveness or high levels of competition for resources. As described previously, in summer and early autumn, juvenile great tits start to show an aggressive behavior more often (Hinde 1952). In flocks, competitive interactions can be observed within and between the sexes, whereby males dominate females in all age classes (Saitou 1979b; Drent 1983). Males also show generally higher levels of aggressive behavior (Saitou 1979b; Drent 1983; Wilson 1992). In various species, females are dominated by males during competitive interactions which can lead to the competitive exclusion of the subordinate sex from preferred resources (Peters and Grubb 1983; Marra 2000; Donald et al. 2007) or can reduce the feeding rates of subordinate age–sex classes (Dunbar and Crook 1975; Benkman 1997). This can eventually lead to segregation of food niches according to sex (Ketterson 1979; Ardia and Bildstein 1997; Marra 2000; Noske 2003; Breed et al. 2006) or can potentially affect sex-specific survival rates (Clobert et al. 1988; Benkman 1997).
Interestingly, juvenile males from female-biased plots were observed closest in the early phase after fledging but farthest in the later phase. Furthermore, a juvenile male’s probability to leave a male-biased plot at least once was lower than for control or female-biased plots. Female-biased plots might thus seem attractive early on potentially because the choice of potential mates is higher or the competition for food is lower. However, later on, juvenile males stay closer in control and male-biased plots than in female-biased plots. We show elsewhere that the local survival of juveniles is higher when they fledged from plots with a high number of same-sex juveniles (Michler et al. 2011). Especially for male juveniles, being with other males of the same age class might increase the chances of establishing a territory under some circumstances because they can put more pressure on the established territory holders (Tinbergen et al. 1987). Therefore, the advantages of being with more males during territory establishment could counteract the negative effects of competitive interactions and cause male young to stay close to the natal plot even or especially when it was previously male-biased (see also Michler et al. 2011 for a more thorough discussion on this topic).
No experimental density effects
We found no overall and no sex-specific effect of the plot density treatment on post-fledging juvenile dispersal. The most likely reason is that the manipulation was relatively small. The relative number of nestlings manipulated was probably also too small compared to the natural variation in density, and therefore the experimental effects may have been swamped by natural variation in density. Furthermore, the two density treatment categories only differed in final nestling densities at day 6 and in fledgling density in one of the two study years (see also “Electronic supplementary material”, Fig. 4). Juvenile males and females did not show differences in dispersal distance or the probability to leave a plot with respect to natural plot densities.
Generally, at high local densities of juveniles, the competition for resources such as food, roosting sites, or territories should increase, and thus we also predicted that high natural plot densities would generate dispersal away from such plots. If, on the other hand, the benefits of high resource abundance counteract the cost of competition, birds should distribute themselves in an ideal free manner and no relation with natural density would be expected. We found that juveniles showed larger distances from plots with high natural densities in the early post-fledging phase than from plots with low natural densities. In our study, the plots with naturally high numbers of young were also the plots with generally high breeding pair densities, and juvenile annual local survival was reduced in such plots (Michler et al. 2011). Furthermore, yearling great tits were less likely to settle in plots with previously high natural densities of young (Nicolaus et al., unpublished data). For various bird species, it was found that juvenile survival rates decrease with increasing population density (Arcese et al. 1992; Perdeck et al. 2000; Nicoll et al. 2003; Armstrong et al. 2005; Dimond and Armstrong 2007) and that the number of juvenile local settlers is negatively related to the density of adults (Van Balen 1980; Drent 1983; Newton and Marquiss 1986). So, the initially larger dispersal distance from natural high density plots suggests that juveniles avoided competition with adults or potentially also with other juveniles in this early phase. The dispersal and movement of juvenile and adults might have reduced the actual densities in the plots in the course of time and thus could explain why the strength of the relationship between natural density and dispersal distance was reduced and almost reversed later in the season (Fig. 4).
Conclusions
Our study showed experimentally that the levels of sex-specific local competition experienced early after fledging affected the juvenile’s dispersal sex-specifically. Juvenile females had a higher probability to leave male-biased plots and had dispersed further from male-biased plots in the later post-fledging phase. Juvenile males were least likely to leave male-biased plots and had smallest dispersal distances from female-biased plots early after fledging. These results are consistent with the idea that, in this early phase after fledging, the individuals reacted sex-specifically to changes in local competition in such a way to maximize their chances of survival. Settlement decisions in a later phase (winter, early spring) may also be governed by sex-specific probabilities of finding a suitable breeding site and partner (Nicolaus et al., unpublished data). Both processes act together to regulate local numbers and sex ratios. Our study suggests that investigating the first steps of the dispersal process is important for a better understanding of the mechanisms and, in combination with an experimental approach, to identify the causal relationships.
References
Arcese P, Smith JNM, Hochachka WM, Rogers CM, Ludwig D (1992) Stability, regulation, and the determination of abundance in an insular song sparrow population. Ecology 73:805–822
Ardia DR, Bildstein KL (1997) Sex-related differences in habitat selection in wintering American kestrels, Falco sparverius. Anim Behav 53:1305–1311
Armstrong DP, Davidson RS, Perrott JK, Roygard J, Buchanan L (2005) Density-dependent population growth in a reintroduced population of North Island saddlebacks. J Anim Ecol 74:160–170
Barrowclough GF (1978) Sampling bias in dispersal studies based on finite area. Bird-Band 49:333–341
Benkman CW (1997) Feeding behavior, flock-size dynamics, and variation in sexual selection in crossbills. Auk 114:163–178
Bennetts RE, Nichols JD, Lebreton JD, Pradel R, Hines JE, Kitchens WM (2001) Methods for estimating dispersal probabilities and related parameters using marked animals. In: Clobert J, Danchin E, Dhondt AA, Nichols JD (eds) dispersal. Oxford University Press, Oxford, pp 3–17
Breed GA, Bowen WD, McMillan JI, Leonard ML (2006) Sexual segregation of seasonal foraging habitats in a non-migratory marine mammal. Proc R Soc London Series B-Biol Sci 273:2319–2326
Clarke AL, Saether BE, Røskaft E (1997) Sex biases in avian dispersal: a reappraisal. Oikos 79:429–438
Clobert J, Perrins CM, McCleery RH, Gosler AG (1988) Survival rate in the great tit Parus major in relation to sex, age, and immigration status. J Anim Ecol 57:287–306
Delestrade A, McCleery RH, Perrins CM (1996) Natal dispersal in a heterogeneous environment: the case of the great tit in Wytham. Acta Oecologica-Int J Ecol 17:519–529
Dhondt AA (1979) Summer dispersal and survival of juvenile great tits in southern Sweden. Oecologia 42:139–157
Dimond WJ, Armstrong DP (2007) Adaptive harvesting of source populations for translocation: a case study with New Zealand robins. Conserv Biol 21:114–124
Dingemanse NJ, Both C, van-Noordwijk AJ, Rutten AL, Drent PJ (2003) Natal dispersal and personalities in great tits (Parus major). Proc R Soc London Series B-Biol Sci 270:741
Dobson FS (1982) Competition for mates and predominant juvenile male dispersal in mammals. Anim Behav 30:1183–1192
Donald PF, Hille S, Brooke MD, Taylor R, Wells CE, Bolton M, Marlow T (2007) Sexual dimorphism, niche partitioning and social dominance in the feeding ecology of the critically endangered Raso lark Alauda razae. Ibis 149:848–852
Drent PJ (1983) The functional ethology of territoriality in the great tit (Parus major L.). University of Groningen, Groningen
Drent PJ (1984) Mortality and dispersal in summer and its consequences for the density of great tits Parus major at the onset of autumn. Ardea 72:127–162
Dunbar RIM, Crook JH (1975) Aggression and dominance in the weaver bird, Quelea quelea. Anim Behav 23:450–459
Feare CJ, Gill EL, McKay HV, Bishop JD (1995) Is the distribution of starlings Sturnus vulgaris within roosts determined by competition. Ibis 137:379–382
Greenwood PJ (1980) Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162
Greenwood PJ, Harvey PH (1982) The natal and breeding dispersal of birds. Ann Rev Ecol Syst 13:1–21
Greenwood PJ, Harvey PH, Perrins CM (1979) The role of dispersal in the great tit (Parus major)—causes, consequences and heritability of natal dispersal. J Anim Ecol 48:123–142
Griffiths R, Double MC, Orr K, Dawson RJ (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Gros A, Hovestadt T, Poethke HJ (2008) Evolution of sex-biased dispersal: the role of sex-specific dispersal costs, demographic stochasticity, and inbreeding. Ecol Model 219:226–233
Hinde RA (1952) The behaviour of the great tit (Parus major) and some other related species. Behav Suppl 2:1–201
Hogstad O (1989) Social-organization and dominance behavior in some Parus species. Wilson Bull 101:254–262
Ketterson ED (1979) Aggressive behavior in wintering dark-eyed juncos—determinants of dominance and their possible relation to geographic variation in sex-ratio. Wilson Bull 91:371–383
Kluyver HN (1951) The population ecology of the great tit, Parus m. major. Ardea 39:1–135
Kluyver HN (1957) Roosting habits, sexual dominance and survival in the great tit. Cold Spring Harb Symp Quant Biol 22:281–285
Marra PP (2000) The role of behavioral dominance in structuring patterns of habitat occupancy in a migrant bird during the nonbreeding season. Behav Ecol 11:299–308
Matthysen E (2005) Density-dependent dispersal in birds and mammals. Ecography 28:403–416
Matthysen E, Van Overveld T, Van de Casteele T, Adriaensen F (2010) Family movements before independence influence natal dispersal in a territorial songbird. Oecologia 162:591–597
Michler SPM (2010) Sex-specific strategies in a sex-biased world. University of Groningen, Groningen
Michler SPM, Nicolaus M, Ubels R et al. (2011) Do sex-specific densities affect local survival of free-ranging great tits? Behav Ecol. doi:10.1093/beheco/arr066
Newton I, Marquiss M (1986) Population regulation in sparrowhawks. J Anim Ecol 55:463–480
Nicolaus M, Michler SPM, Ubels R, van der Velde M, Komdeur J, Both C, Tinbergen JM (2009) Sex-specific effects of altered competition on nestling growth and survival: an experimental manipulation of brood size and sex ratio. J Anim Ecol 78:414–426
Nicoll MAC, Jones CG, Norris K (2003) Declining survival rates in a reintroduced population of the Mauritius kestrel: evidence for non-linear density dependence and environmental stochasticity. J Anim Ecol 72:917–926
Noske RA (2003) Sexual differences in the foraging behaviour of crested shrike-tits, Falcunculus frontatus, during winter. EMU 103:271–277
Perdeck AC, Visser ME, Van Balen JH (2000) Great tit Parus major survival, and the beech-crop cycle. Ardea 88:99–108
Perrin N, Mazalov V (2000) Local competition, inbreeding, and the evolution of sex-biased dispersal. Am Nat 155:116–127
Peters WD, Grubb TC (1983) An experimental analysis of sex-specific foraging in the downy woodpecker, Picoides pubescens. Ecology 64:1437–1443
Pusey AE (1987) Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends Ecol Evol 2:295–299
Rasbash J, Steele F, Browne W et al (2004) A user's guide to MLwiN, version 2.0. Centre for Multilevel Modelling, Institute of Education, University of London, London
Richardson K, Ewen JG, Armstrong DP, Hauber ME (2010) Sex-specific shifts in natal dispersal dynamics in a reintroduced hihi population. Behaviour 147:1517–1532
Saitou T (1979a) Ecological study of social organization in the great tit (Parus major) II. Formation of the basic flock. J Yamashina Inst Ornithol 11:137–148
Saitou T (1979b) Ecological study of social organization in the great tit (Parus major) III. Home range of the basic flocks and dominance relationship of the members of basic flocks. J Yamashina Inst Ornithol 11:149–171
St. Louis VL, Barlow JC, Sweerts J-PRA (1989) Toenail-clipping: a simple technique for marking individual nidicolous chicks. J Field Ornithol 60:211–215
Summers RW, Westlake GE, Feare CJ (1986) Differences in the ages, sexes and physical condition of starlings (Sturnus vulgaris) at the centre and periphery of roost. Ibis 129:96–102
Tarvin KA, Woolfenden GE (1997) Patterns of dominance and aggressive behavior in blue jays at a feeder. Condor 99:434–444
Tinbergen JM (2005) Biased estimates of fitness consequences of brood size manipulation through correlated effects on natal dispersal. J Anim Ecol 74:1112–1120
Tinbergen JM, van Balen JH, Drent PJ, Cavé AJ, Mertens JAL, Den Boer-Hazewinkel J (1987) Population dynamics and cost–benefit analysis—an attempt to relate population dynamics via lifetime reproductive success to short-term decisions. Netherlands J Zool 37:180–213
Van Balen JH (1980) Population fluctuations of the great tit and feeding conditions in winter. Ardea 68:143–164
Verhulst S, Hut RA (1996) Post-fledging care, multiple breeding and the costs of reproduction in the great tit. Anim Behav 51:957–966
Verhulst S, Perrins CM, Riddington R (1997) Natal dispersal of great tits in a patchy environment. Ecology 78:864–872
Walsh PS, Metzger DA, Higuchi R (1991) Chelex-100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10:506–513
Wild G, Taylor PD (2004) Kin selection models for the co-evolution of the sex ratio and sex-specific dispersal. Evol Ecol Res 6:481–502
Wilson JD (1992) Correlates of agonistic display by great tits Parus major. Behaviour 121:168–214
Wilson AG, Arcese P (2008) Influential factors for natal dispersal in an avian island metapopulation. J Avian Biol 39:341–347
Acknowledgements
This study was part of SPMM and MN's Ph.D. work. RU assisted in field work and managed the database. MvdV performed the molecular sexing of the nestlings. CB and JMT collected data in the field and assisted in planning and discussion as did JK. We thank Luc te Marvelde and Kim Meijer for assistance in the lab. Karen Bouwman and Niels Dingemanse were very valuable in discussion and in the field. We are grateful to Martin Keiser, Tamar Lok, Jeroen Reimerink, and Kirsten Jalvingh as well as to all the Animal Ecology course students in 2005 and 2006 for their help in collecting the data. Finally, we thank “Staatsbosbeheer” and the Royal Dutch army “Koninklijke Landmacht” for giving us their permission to work in the Lauwersmeer area and to stay in the army base. This work was supported by a grant from the Netherlands Organisation for Scientific Research (NWO-VICI, 86503003) awarded to JK as well as by the University of Groningen.
Ethical standards
The experiments complied with the current Dutch law (DEC nr. 4114B).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Gibson
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Michler, S.P.M., Nicolaus, M., Ubels, R. et al. Sex-specific effects of the local social environment on juvenile post-fledging dispersal in great tits. Behav Ecol Sociobiol 65, 1975–1986 (2011). https://doi.org/10.1007/s00265-011-1207-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1207-1