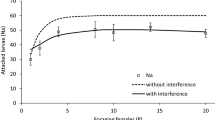
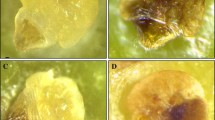
Abstract
Natural enemies exert selection pressure on their prey. Predators and parasitoids drive their prey into the evolution of novel traits to cope with this stress. When eggs and juveniles are the target of enemies, defense strategies may rely on adults. However, it is not easy to predict whether adults should actively protect unrelated offspring. Females of the golden egg bug (Phyllomorpha laciniata) mainly oviposit on conspecifics of either sex. Females can also lay eggs on their food plant. Eggs placed on plants suffer from a higher mortality caused by natural enemies than eggs carried by bugs. Females never carry their own eggs and males are seldom related to the eggs they carry. We experimentally explored if conspecifics protect the eggs by studying the behavioral interaction between P. laciniata individuals and the specialist egg parasitoid Gryon bolivari. All bugs exhibited active responses against parasitoids regardless of the sex of the bug, the egg load, and their mating status. Most of the responses prevented parasitoids from reaching the eggs, and thus they reduced the risk of egg parasitization. Although responses of bugs were effective to overcome parasitoid attacks, we suggest that egg protection against parasitoids has evolved as a co-opted trait from a general defense of adult bugs against enemies. In this system, egg defense is not an individual's strategy to protect the offspring, but rather a consequence of the eggs being attached to one's body. It may also explain the low parasitization carried eggs suffer in the wild. The results further highlight the idea of conspecifics as an enemy-free space in P. laciniata.




Similar content being viewed by others
References
Blum MS, Hilker M (2002) Chemical protection of insect eggs. In: Hilker M, Meiners T (eds) Chemoecology of insect eggs and egg deposition. Blackwell, Oxford, pp 61–90
Broström G (2008) glmmML: Generalized linear models with clustering. R package version 0.81-3. http://cran.r-project.org/
Carrasco D, Kaitala A (2009) Egg-laying tactic in Phyllomorpha laciniata in the presence of parasitoids. Entomol Exp Appl 131:300–307
Carrasco D, Borg AA, Kaitala A (2007) Egg-laying in relation to egg substrate in Gryon bolivari, an egg parasitoid of the golden egg bug (Phyllomorpha laciniata). J Insect Behav 20:307–313
Cocroft RB (2002) Antipredator defense as a limited resource: unequal predation risk in broods of an insect with maternal care. Behav Ecol 13:125–133
Danks HV (2002) Modification of adverse conditions by insects. Oikos 99:10–24
Eberhard W (1975) The ecology and behavior of a subsocial pentatomid bug and two scelionid wasps: strategy and counterstrategy in a host and its parasites. Smithson Contrib Zool 205:1–39
Evans L, Schmidt O (eds) (1990) Insect defenses: adaptive mechanisms and strategies of prey and predators. State University of New York Press, New York
Fatouros NE, Dicke M, Mumm R, Meiners T, Hilker M (2008) Foraging behavior of egg parasitoids exploiting chemical information. Behav Ecol 19:677–689
Godfray HCJ (1994) Parasitoids: behavioral and evolutionary ecology. Princeton Univ Press, New Jersey
Gomendio M, Garcia-Gonzalez F, Reguera P, Rivero A (2008) Male egg carrying in Phyllomorpha laciniata is favoured by natural not sexual selection. Anim Behav 75:763–770
Goubault M, Scott D, Hardy ICW (2007) The importance of offspring value: maternal defence in parasitoid contests. Anim Behav 74:437–446
Gross P (1993) Insect behavioral and morphological defenses against parasitoids. Annu Rev Entomol 38:251–273
Jeffries MJ, Lawton JH (1984) Enemy free space and the structure of ecological communities. Biol J Linn Soc 23:269–286
Kaitala A (1996) Oviposition on the back of conspecifics: an unusual reproductive tactic in a coreid bug. Oikos 77:381–389
Kaitala A, Miettinen M (1997) Female egg dumping and the effect of sex ratio on male egg carrying in a coreid bug. Behav Ecol 8:429–432
Kaitala A, Espadaler X, Lehtonen R (2000) Ant predation and the cost of egg carrying in the golden egg bug: experiments in the field. Oikos 89:254–258
Kudo S, Ishibashi E (1996) Maternal defence of a leaf beetle is not effective against parasitoids but is against pedestrian predators. Ethology 102:560–567
Mappes J, Kaitala A (1994) Experiments with Elasmucha grisea L. (Heteroptera: Acanthosomatidae): does a female parent bug lay as many eggs as she can defend? Behav Ecol 5:314–317
McCulloch CE, Searle SR (2001) Generalized, linear, and mixed models. Wiley, New York
Miettinen M, Kaitala A, Espadaler X, Tannerfeldt M (2004) The effect of ant predation on survival and mating frequency of the golden egg bug in field experiments (Hymenoptera: Formicidae; Heteroptera: Coreidae). Sociobiology 44:659–668
Miettinen M, Kaitala A, Smith RL, Macías Ordóñez R (2006) Do egg carrying and protracted copulation affect mobility in the golden egg bug? J Insect Behav 19:171–178
Nakahira T, Kudo S (2008) Maternal care in the burrower bug Adomerus triguttulus: defensive behavior. J Insect Behav 21:306–316
Nomikou M, Janssen A, Sabelis MW (2003) Herbivore host plant selection: whitefly learns to avoid host plants that harbour predators of her offspring. Oecologia 136:484–488
R Development Core Team (2008) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Reguera P, Gomendio M (2002) Flexible oviposition behavior in the golden egg bug (Phyllomorpha laciniata) and its implications for offspring survival. Behav Ecol 13:70–74
Tallamy DW, Denno RF (1981) Maternal care in Gargaphia solani (Hemiptera, Tingidae). Anim Behav 29:771–778
Tallamy DW, Wood TK (1986) Convergence patterns in subsocial insects. Annu Rev Entomol 31:369–390
Tallamy DW, Schaefer C (1997) Maternal care in the Hemiptera: ancestry, alternatives, and current adaptive value. In: Choe JC, Crespi BJ (eds) The evolution of social behavior in insects and arachnids. Cambridge University Press, Cambridge, pp 94–115
Tay WT, Miettinen M, Kaitala A (2003) Do male golden egg bugs carry eggs they have fertilized? A microsatellite analysis. Behav Ecol 14:481–485
Venables WN, Ripley BD (1999) Modern applied statistics with S-PLUS. Springer, New York
Vet LEM, Groenewold AW (1990) Semiochemicals and learning in parasitoids. J Chem Ecol 16:3119–3135
Zeileis A, Hothorn T (2002) Diagnostic checking in regression relationships. R News. 2/3:7-10. http://cran.r-project.org/
Acknowledgments
S. Kivelä, P. Välimäki, S. Varga, C. Nuortilla, J.C. Illera, and anonymous reviewers provided helpful, valuable, and constructive comments on the manuscript. We are also grateful to S. Kivelä for statistical advice and S. Varga for field work assistance. This work has been supported by the Finnish Cultural Foundation, Alfred Kordelin Foundation and Oskar Öflunds Foundation [grants to D.C.]. The authors declare that they have no conflict of interest.
The experiments performed comply with the current laws of Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Gwynne
Rights and permissions
About this article
Cite this article
Carrasco, D., Kaitala, A. Active protection of unrelated offspring against parasitoids. A byproduct of self defense?. Behav Ecol Sociobiol 64, 1291–1298 (2010). https://doi.org/10.1007/s00265-010-0943-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-0943-y