Abstract
Purpose
Osteoporosis is a problem for many patients after total knee arthroplasty (TKA). The aseptic loosening of the prosthesis is also a significant problem. Therefore, in these patients, bisphosphonates (BPs) are used that, by influencing the level of bone turnover markers, reduce the risk of osteoporotic fractures and aseptic revisions in TKA. The purpose of the study was to assess whether the Pamifos® present in bone cement has any effect on the level of selected bone turnover markers and cytokines in patients after total knee arthroplasty.
Methods
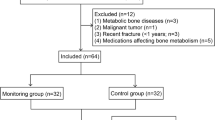
The study group consisted of 30 women with degenerative changes of the knee joint, whose total knee prosthesis was stabilized with cement enriched with Pamifos®. The control group consisted of 30 women treated for degenerative changes of the knee joint without the use of bisphosphonate-enriched cement for prosthetic stabilization.
Results
In the study group, we found a decrease in tumour necrosis factor (TNF-α) levels 12 weeks after surgery, whereas the control group experienced an almost twofold increase in TNF-α level. The concentration of OPG, a natural RANKL antagonist, was highest in patients of the study group six weeks after surgery and was four times higher compared to the control group. Statistically significant differences were found in the RANKL level (P < 0.05). In the control group, there was a continuous increase in RANKL concentration from the first to the 12th week after surgery. The highest level of RANKL in patients of the study group was found six weeks after the surgery, and 12 weeks after knee arthroplasty, it was significantly lower. It was found that the concentration of osteocalcin (OC) in the study group was the lowest three weeks after the surgery, then it increased and remained at a similar level after 12 weeks. The concentrations of selected cytokines (IL-1β, IL-2, IL-6, IL-10, IL-17AF) also showed statistically significant differences.
Conclusions
The BP-stimulated increase in the level of OPG and the decrease in the level of RANKL, as well as the impact on the level of the analyzed interleukins in the bone microenvironment, may be an important element of the mechanisms limiting bone resorption. Therefore, the use of BP-enriched cement implants appears to be justified.









Similar content being viewed by others
Data availability
All personal patients’ data are available at Orthopedic Department of St Johns’ Oncology Center in Lublin, Poland. Biochemical results are available at Department of Biochemistry and Biotechnology, Institute of Biological Sciences, Maria Curie-Sklodowska University, Lublin, Poland.
References
Harris WH, Sledge CB (1990) Total hip and total knee replacement. N Engl J Med 323:725–731
Nyman JS, Rodrigo JJ, Hazelwood SJ, Yeh OC, Martin RB (2006) Predictions on preserving bone mass in knee arthroplasty with bisphosphonates. J Arthroplasty 21:1106–1113
Kennel KA, Drake MT (2009) Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clin Proc 84(7):632–638. https://doi.org/10.1016/S0025-6196(11)60752-0
Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C (2006) Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop 77(2):177–197. https://doi.org/10.1080/17453670610045902
Ingham E, Fisher J (2005) The role of macrophages in osteolysis of total joint replacement. Biomaterials 26:1271–1286. https://doi.org/10.1016/j.biomaterials.2004.04.035
Bitar D, Parvizi J (2015) Biological response to prosthetic debris. World J Orthop 6:172–189. https://doi.org/10.5312/wjo.v6.i2.172
Tateiwa D, Yoshikawa H, Kaito T (2019) Cartilage and bone destruction in arthritis: pathogenesis and treatment strategy: a literature review. Cells 2;8(8):E818. https://doi.org/10.3390/cells8080818.
Mannino MH, Zhu Z, Xiao H et al (2015) The paradoxical role of IL-10 in immunity and cancer. Cancer Lett 367(2):103–107. https://doi.org/10.1016/j.canlet.2015.07.009
Shi J, Liang G, Huang R, Liao L, Qin D (2018) Effects of bisphosphonates in preventing periprosthetic bone loss following total hip arthroplasty: a systematic review and meta-analysis. J Orthop Surg Res 13:225
Mazurkiewicz T, Matuszewski Ł, Matuszewska A, Jaszek M (2013) Implanted bisphosphonates in bone cement affect bonemarkers in rat serum. Int Orthop 37:969–974. https://doi.org/10.1007/s00264-013-1816-6
Matuszewski L, Olchowik G, Mazurkiewicz T, Kowalczyk B, Zdrojewska A, Matuszewska A, Ciszewski A, Gospodarek M, Morawik I (2013) Biomechanical parameters of the BP-enriched bone cement. Europ J of Ort/op Surg and Traumatol 24(4):435–441. https://doi.org/10.1007/s00590-013-1230-1
Matuszewski L, Turzańska K, Matuszewska A, Jabłoński M, Polkowska I, Mazurkiewicz T (2013) Effect of implanted bisphosphonate-enriched cement on the trabecular microarchitecture of bone in a rat model using micro-computed tomography. Int Ort/op 37(6):1187–1193. https://doi.org/10.1007/s00264-013-1855-z
Guo EW, Sayeed Z, Padela MT, Qazi M, Zekaj M, Schaefer P, Darwiche HF (2018) Improving total joint replacement with continuous quality improvement methods and tools. Orthop Clin North Am 49(4):397–403. https://doi.org/10.1016/j.ocl.2018.05.002
Segal JB, Eng J, Tamariz LJ, Bass EB (2003) Review of the evidence on diagnosis of deep venous thrombosis and pulmonary embolism. Ann Fam Med 5:6373. https://doi.org/10.1370/afm.648
Ambroszkiewicz J, Gajewska J, Chełchowska M, Ołtarzewski M, Laskowska-Klita T, Nowacka M, Milanowski A (2008) Concentration of osteoprotegerin, bone formation and resorption markers in patients with phenylketonuria. Pol Merkur Lekarski XXV 145:57
Price PA, Parthemore JG, Deftos LJ (1980) New biochemical marker for bone metabolism: measurement by radioim- munoassay of bone GLA-protein in the plasma of normal subjects and patients with bone disease. J Ctin Invest 66:878–883
Hofbauer LC, Heufelder AE (2000) The role of receptor activator of nuclear factor -(B ligand and osteoprotegerin in the pathogenesis and treatment of metabolic bone diseases. J Clin Endocrinol Metab 85:2355–2363
Udagawa N, Takahashi N, Yasuda H et al (2000) Osteoprotegerin produced by osteoblasts is an important regulator in osteoc last development and function. Endocrinol 141:3478–3484
Nicole YC, Schindeler A, Tägil M, Ruys AJ, Little DG (2012) Use of BMPs and bisphosphonates to improve bone fracture healing. Frontiers in Bioscience E 4:26472653
Jakobsen T, Baas J, Bechtold JE, Elmengaard B, Soballe K (2007) Soaking morselized allograft in bisphosphonate can impair implant fixation. Clin Orthop Relat Res 463:195–201
Li P et al (2004) RANK signaling is not required for TNFα- mediated in-crease in CD11hi osteoclast precursors but is essential for mature osteoclast formation in TNFα-mediated inflammatory arthritis. J Bone Miner Res 19:207–213
Kurihara N, Bertolini D, Suda T, Akiyama Y, Roodman GD (1990) IL-6 stimulates osteoclast-like multinucleated cel formation in long term human marrow cultures by inducing IL-1 release. J Immunol 144:4226–4230
McInnes I, Schett G (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nat Immunol 7:429–442
Redlich K, Smolen JS (2012) Inflammatory bone loss: pathogenesis and therapeutic intervention. Nature Rev Drug Discov 11(3):234–250
Steeve KT, Marc P, Sandrine T, Dominique H, Yannick F (2004) IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysio-logy. Cytokine & Growth Fac Rev 15(1):49–60
Głuszko P (2009) Effects of biologic antirheumatic treatments on bone metabolismin rheumatoid arthritis and ankylosing spondylitis. Polish J Endocrinol 60; 2/2009ISSN 0423–104X, 115–121.
Sarkar S, Justa S, Brucks M, Endres J, Fox DA, Zhou X, Alnaimat F, Whitaker B, Wheeler JC, Jones BH, Bommireddy SR (2014) Interleukin (IL)-17A, F and AF in inflammation: a study in collagen-induced arthritis and rheumatoid arthritis. Clin Exp Immunol 177(3):652–661
van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA (2011) Inflammasome activation and IL-1beta and IL-18 processing during infection Trends Immunol 32:110–116.
Fiorentino D, Bond M, Mosmann T (1989) Two types of mouse T helpercell. IV. Th2 clones secrete a factor that inhibits cytokine productionby Th1 clones. J Exp Med 170:2081–2095
De Waal MR, Figdor C, Huijbens R, Mohan-Peterson S, Bennett B, Culpepper J, Dang W, Zurawski G, De Vries J (1993) Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN–gamma or IL-10. J Immunol 151:6370–6381
Kang K, Im S (2005) Differential regulation of the IL-10 gene in Th1and Th2 T cells. Ann NY Acad Sci 1050:97–107
Little D, Cornell M, Hile M, Briody J, Cowell C, Bilston L (2001) Effect of pamidronate on distraction osteogenesis and fixator-related osteoporosis. Injury 32:1420
Schindeler A, Little D (2007) Bisphosphonate action: revelations and deceptions from in vitro studies. J Pharm Sci 96:1872–1878
Shi J, Liang G, Huang R, Liao L, Qin D (2018) Effects of bisphosphonates in preventing periprosthetic bone loss following total hip arthroplasty: a systematic review and metaanalysis. Orthop Surg and Res 13:225. https://doi.org/10.1186/s13018-018-0918-7
Author information
Authors and Affiliations
Contributions
Each author has contributed substantially to the submitted work and has reviewed and agrees with the submission of the manuscript for review.
AM: research idea, prepared the manuscript, biochemic analysis, and statistical analysis.
ŁM: prepared the manuscript, patient’s selection, and surgical procedures.
MJ: biochemic analysis.
PP and SS: surgical procedures.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the institutional Ethics Committees of the Medical University in Lublin.
Consent to participate
All authors have read and approved the final draft.
Consent for publication
All authors have made a significant contribution to the findings and methods in the paper.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matuszewska, A., Matuszewski, Ł., Jaszek, M. et al. Effect of bisphosphonates on selected markers of bone turnover in patients after total knee arthroplasty. International Orthopaedics (SICOT) 46, 1529–1538 (2022). https://doi.org/10.1007/s00264-022-05407-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-022-05407-z