Abstract
Purpose
Stromal vascular fraction (SVF) as an injectable regenerative therapy for knee osteoarthritis (OA) has gained recent popularity. However, there is no clear consensus on the outcomes of such treatment. We systematically reviewed available evidence on the use of SVF injection in the treatment of knee OA.
Methods
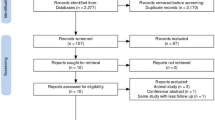
The study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, with keyword search in PubMed, Scopus, and the Cochrane Library Database and related article search in Google Scholar. Clinical studies demonstrating effects of SVF in knee OA and published in English literature were included. Risk of bias assessment was done with modified Coleman Methodology Scoring (CMS).
Results
Eleven studies (9 prospective, 2 retrospective) that contributed to 290 knees in 200 patients were included. Two studies that contributed to 3718 knee injections were excluded from pooled analysis and were scrutinized separately. Majority of patients reported improvement in pain, range of motion (ROM), functional rating, six metre walking distance, and functional outcome scores. There was no major donor-site morbidity. There was only one reported case of knee joint infection and no case of tumour formation in relation to SVF injection.
Discussion
Intra-articular injection of SVF can be a simple, affordable, and minimally invasive treatment that could serve as an interim option for patients who failed other conservative and arthroscopic options.
Conclusion
Intra-articular injection of SVF is a safe and effective technique for the management of knee OA. However, comparative Level I studies are needed to support the use of adjuvants with SVF and also to compare the use of SVF (with or without adjuvants) with ADMSCs, PRP, and bone marrow concentrate.



Similar content being viewed by others
Data availability
Compilation of data extracted from the studies of the systematic review is available for submission when needed.
References
Arden NK, Leyland KM (2013) Osteoarthritis year 2013 in review: clinical. Osteoarthritis Cartilage 21:1409–1413
Papalia R, Di Pino G, Tecame A et al (2015) Biomechanical and neural changes evaluation induced by prolonged use of non-stable footwear: a systematic review. Musculoskelet Surg 99:179–187
Gao SG, Li KH, Zeng KB et al (2010) Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthritis Cartilage 18:82–87
Zhang W, Nuki G, Moskowitz RW et al (2010) OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 18:476–499
Dieppe P, Lim K, Lohmander S (2011) Who should have knee joint replacement surgery for osteoarthritis? Int J Rheum Dis 14:175–180
Gibbs N, Diamond R, Sekyere EO et al (2015) Management of knee osteoarthritis by combined stromal vascular fraction cell therapy, platelet-rich plasma, and musculoskeletal exercises: a case series. J Pain Res 8:799–806. https://doi.org/10.2147/JPR.S92090
Riordan NH, Ichim TE, Min WP et al (2009) Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med 7:29
Berman, M., Lander, E., Grogan, T., et al. (2019) Prospective study of autologous adipose derived stromal vascular fraction containing stem cells for the treatment of knee osteoarthritis. Int J Stem Cell Res Ther 6:064. https://doi.org/10.23937/2469-570X/1410064
Moher, D., Liberati, A., Tetzlaff, J., et al. The PRISMA Group (2009), Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, PLoS Medicine, vol. 6, no. 7, article e1000097.
Kon E, Verdonk P, Condello V et al (2009) Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. The American Journal of Sports Medicine 37(Supplement 1):156–166
Abdul-Wahab TA, Betancourt JP et al (2016) Initial treatment of complete rotator cuff tear and transition to surgical treatment: systematic review of the evidence. Muscles Ligaments Tendons J 6:35–47. https://doi.org/10.11138/mltj/2016.6.1.035
Michalek, J., Vrablikova, A. , Heinrich, K.G., et al. (2019) Stromal vascular fraction cell therapy for a stroke patient-cure without side effects. Brain Sciences; 9(3). 10.3390/brainsci9030055.
Pak J (2011) Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J Med Case Rep 5:296
Bui KH, Duong TD, Nguyen TN et al (2014) Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: a clinical study. Biomed Res Ther 1:2–8
Garza JR, Santa Maria D, Palomera T et al (2015) Use of autologous adipose-derived stromal vascular fraction to treat osteoarthritis of the knee: a feasibility and safety study. J Regen Med 4(1). https://doi.org/10.4172/2325-9620.1000119
Al-Salahat N (2016) Pre-SVF arthroscopy: a case report of new concept of meniscus and cartilage regeneration using arthroscopy followed by intra-articular injection of adipose-derived stromal vascular fraction. Stem Cell Biol Res 3:2. https://doi.org/10.7243/2054-717X-3-2
Fodor PB, Paulseth SG (2015) Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthetic Surgery Journal 36(2):229–236. https://doi.org/10.1093/asj/sjv135
Pak J, Lee JH, Park KS et al (2016) Regeneration of cartilage in human knee osteoarthritis with autologous adipose tissue-derived stem cells and autologous extracellular matrix. Biores Open Access 5(1):192–200. https://doi.org/10.1089/biores.2016.0024
Bansal H, Comella K, Leon J et al (2017) Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J Transl Med 15:141. https://doi.org/10.1186/s12967-017-1242-4
Yokota N, Yamakawa M, Shirata T et al (2017) Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regen Ther 6:108–112. https://doi.org/10.1016/j.reth.2017.04.002
Lapuente JP, Dos-Anjos S, Blázquez-Martínez A (2020) Intra-articular infiltration of adipose-derived stromal vascular fraction cells slows the clinical progression of moderate-severe knee osteoarthritis: hypothesis on the regulatory role of intra-articular adipose tissue. Journal of Orthopaedic Surgery and Research 15(1):137. https://doi.org/10.1186/s13018-020-01664-z
Tsubosaka, M., Matsumoto, T., Sobajima, S., et al. (2020) The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskelet Disord, 21, 207. https://doi.org/10.1186/s12891-020-03231-3
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957(16):494–502
Pers YM, Rackwitz L, Ferreira R et al (2016) Adipose Mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med 5:847–856
Hudetz D, Borić I, Rod E et al (2019) Early results of intra-articular micro-fragmented lipoaspirate treatment in patients with late stages knee osteoarthritis: a prospective study. Croat Med J 60(3):227–236. https://doi.org/10.3325/cmj.2019.60.227
Russo A, Screpis D, Di Donato SL et al (2018) Autologous micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis: an update at 3 year follow-up. J Exp Orthop 5:52. https://doi.org/10.1186/s40634-018-0169-x
Roato I, Belisario DC, Compagno M et al (2019) Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: clinical and histological observations. International Orthopaedics (SICOT) 43:15–23. https://doi.org/10.1007/s00264-018-4192-4
Koh YG, Jo SB (2013) Mesenchymal stem cells injections improve symptoms of knee osteoarthritis. Arthroscopy 29(4):748–755
Hurley ET, Yasui Y, Gianakos AL et al (2018) Limited evidence for adipose-derived stem cell therapy on the treatment of osteoarthritis. Knee Surg Sports Traumatol Arthrosc 26(11):3499–3507. https://doi.org/10.1007/s00167-018-4955-x
Cavallo C, Boffa A, Andriolo L et al (2020) Bone marrow concentrate injections for the treatment of osteoarthritis: evidence from preclinical findings to the clinical application. International Orthopaedics (SICOT). https://doi.org/10.1007/s00264-020-04703-w
Marenah, M., Li, J., Kumar, A., et al. (2019). Quality assurance and adverse event management in regenerative medicine for knee osteoarthritis: current concepts. Journal of clinical orthopaedics and trauma, 10(1), 53–58. https://doi.org/10.1016/j.jcot.2018.09.005
Anz AW, Bapat A, Murrell WD (2016) Concepts in regenerative medicine: past, present, and future in articular cartilage treatment. Journal of clinical orthopaedics and trauma 7(3):137–144. https://doi.org/10.1016/j.jcot.2016.05.006
“Regulatory considerations for human cells, tissues, and cellular and tissue-based products: minimal manipulation and homologous use” FDA Guidance. July 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-human-cells-tissues-and-cellular-and-tissue-based-products-minimal
Di Matteo B, Vandenbulcke F, Vitale ND et al (2019) Minimally manipulated mesenchymal stem cells for the treatment of knee osteoarthritis: a systematic review of clinical evidence. Stem Cells Int 1735242:2019. https://doi.org/10.1155/2019/1735242
Tiwari SS, Raman S, Martin P (2017) Regenerative medicine in India: trends and challenges in innovation and regulation. Regen Med. 12(7):875–885. https://doi.org/10.2217/rme-2017-0094
Pak J, Lee J, Pak N et al (2018) Cartilage regeneration in humans with adipose tissue-derived stem cells and adipose stromal vascular fraction cells: updated status. International Journal of Molecular Sciences 19(7):2146
Pak J, Chang JJ, Lee LH (2013) Safety reporting on implantation of autologous adipose tissue-derived stem cells with platelet-rich plasma into human articular joints. BMC Musculoskelet Disord 14:337
Pagano C, Calcagno A, Giacomelli L et al (2004) Molecular and morphometric description of adipose tissue during weight changes: a quantitative tool for assessment of tissue texture. Int J Mol Med 14:897–902
Victor, S. (2014) Isolation of stromal vascular fraction from adipose tissue obtained from postmortem source using ultrasonic cavitation. WO 2014015229 A1.
Amirkhani MA, Mohseni R, Soleimani M et al (2016) A rapid sonication based method for preparation of stromal vascular fraction and mesenchymal stem cells from fat tissue. Bioimpacts 6(2):99–104. https://doi.org/10.15171/bi.2016.14
Aronowitz JA, Lockhart RA, Hakakian CS et al (2015) Clinical safety of stromal vascular fraction separation at the point of care. Ann Plast Surg 75(6):666–671. https://doi.org/10.1097/SAP.0000000000000594
Lee, C.S., Burnsed, O.A., Raghuram, V., et al. (2012) Adipose stem cells can secrete angiogenic factors that inhibit hyaline cartilage regeneration. Stem cell research & therapy, 24;3(4):35.
Van Pham P, Bui KH, Ngo DQ et al (2013a) Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem cell research & therapy 4:91
Hong Z, Chen J, Zhang S et al (2019) Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. Int Orthop 43:1123–1134
Author information
Authors and Affiliations
Contributions
Conceptualization: Dr. WDM, Dr. RV
Data curation: Dr. SS, Dr. AV, Dr. VC
Supervision: Dr. WDM, Dr. RV
Statistical analysis: Dr. VC
Writing original draft: Dr. SS, Dr. VC, Dr. AV
Critical review: Dr. WDM, Dr. RV
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable as it is a systematic review and not a research paper.
Consent to participate
Not applicable as it is a systematic review and did not involve direct patient care.
Consent to publish
Not applicable as it is a systematic review and did not involve direct patient care.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Level of evidence: Level IV, systematic review of levels II–IV studies
Rights and permissions
About this article
Cite this article
Shanmugasundaram, S., Vaish, A., Chavada, V. et al. Assessment of safety and efficacy of intra-articular injection of stromal vascular fraction for the treatment of knee osteoarthritis—a systematic review. International Orthopaedics (SICOT) 45, 615–625 (2021). https://doi.org/10.1007/s00264-020-04926-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-020-04926-x