Abstract
Purpose
Although there are various new scaffold-based techniques for cartilage regeneration it remains unclear up to which defect size they can be used. The present study reports of a cell-free collagen type I gel matrix for the treatment of large cartilage defects of the knee after a two-year follow-up.
Methods
Twenty-eight patients with a mean cartilage defect size of 3.71 ± 1.93 cm² were treated with a cell-free collagen type I gel matrix (CaReS-1S®, Arthro Kinetics AG, Krems/Donau, Austria) via a mini-arthrotomy. Clinical outcome was assessed preoperatively and six weeks as well as six, 12 and 24 months after surgery using various clinical outcome scores (IKDC, Tegner, KOOS, VAS). Cartilage regeneration was evaluated via MRI using the MOCART score.
Results
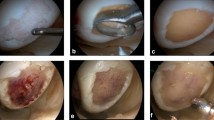
Seventeen male and 11 female patients with a mean age of 34.6 years were included in this study. Significant pain reduction (VAS) could be noted after six weeks already. Patient activity (IKDC, Tegner) could be significantly improved from 12 months on and nearly reached reported pre-operative values. All subject categories of the KOOS except for symptom (swelling) showed significant improvements throughout the study. Constant significant improvements of the mean MOCART score were observed from 12 months on. MR images did not yield any signs of infection or synovitis. After 24 months a complete defect filling could be noted in 24 out of 28 cases with a mainly smooth surface, complete integration of the border zone and homogenous structure of the repaired tissue.
Conclusion
Cell-free collagen type I matrices appear to be a safe and suitable treatment option even for large cartilage defects of the knee. Results of this study were comparable to the better-established findings for small cartilage defects. Mid- and long-term results will be needed to see if clinical and MR-tomographic outcome can be maintained beyond 24 months.



Similar content being viewed by others
References
Andereya S, Maus U, Gavenis K, Müller-Rath R, Miltner O, Mumme T, Schneider U (2006) First clinical experiences with a novel 3D-collagen gel (CaReS) for the treatment of focal cartilage defects in the knee. Z Orthop Ihre Grenzgeb 144:272–280
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Chiang H, Liao C-J, Hsieh C-H, Shen C-Y, Huang Y-Y, Jiang C-C (2013) Clinical feasibility of a novel biphasic osteochondral composite for matrix-associated autologous chondrocyte implantation. Osteoarthritis Cartilage 21:589–598
Ebert JR, Fallon M, Zheng MH, Wood DJ, Ackland TR (2012) A randomized trial comparing accelerated and traditional approaches to postoperative weightbearing rehabilitation after matrix-induced autologous chondrocyte implantation: findings at 5 years. Am J Sports Med 40:1527–1537
Efe T, Füglein A, Heyse TJ, Stein T, Timmesfeld N, Fuchs-Winkelmann S, Schmitt J, Paletta JRJ, Schofer MD (2012) Fibrin glue does not improve the fixation of press-fitted cell-free collagen gel plugs in an ex vivo cartilage repair model. Knee Surg Sports Traumatol Arthrosc 20:210–215
Efe T, Getgood A, Schofer MD, Fuchs-Winkelmann S, Mann D, Paletta JRJ, Heyse TJ (2012) The safety and short-term efficacy of a novel polyurethane meniscal scaffold for the treatment of segmental medial meniscus deficiency. Knee Surg Sports Traumatol Arthrosc 20:1822–1830
Efe T, Theisen C, Fuchs-Winkelmann S, Stein T, Getgood A, Rominger MB, Paletta JRJ, Schofer MD (2012) Cell-free collagen type I matrix for repair of cartilage defects-clinical and magnetic resonance imaging results. Knee Surg Sports Traumatol Arthrosc 20:1915–1922
Enea D, Cecconi S, Busilacchi A, Manzotti S, Gesuita R, Gigante A (2012) Matrix-induced autologous chondrocyte implantation (MACI) in the knee. Knee Surg Sports Traumatol Arthrosc 20:862–869
Erdil M, Bilsel K, Taser OF, Sen C, Asik M (2013) Osteochondral autologous graft transfer system in the knee; mid-term results. Knee 20:2–8
Filardo G, Kon E, Perdisa F, Balboni F, Marcacci M (2014) Autologous osteochondral transplantation for the treatment of knee lesions: results and limitations at two years’ follow-up. Int Orthop 38:1905–1912
Flandry F, Hunt JP, Terry GC, Hughston JC (1991) Analysis of subjective knee complaints using visual analog scales. Am J Sports Med 19:112–118
Frenkel SR, Clancy RM, Ricci JL, Di Cesare PE, Rediske JJ, Abramson SB (1996) Effects of nitric oxide on chondrocyte migration, adhesion, and cytoskeletal assembly. Arthritis Rheum 39:1905–1912
Gobbi A, Karnatzikos G, Kumar A (2014) Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc 22:1986–1996
Gosiewska A, Rezania A, Dhanaraj S, Vyakarnam M, Zhou J, Burtis D, Brown L, Kong W, Zimmerman M, Geesin JC (2001) Development of a three-dimensional transmigration assay for testing cell-polymer interactions for tissue engineering applications. Tissue Eng 7:267–277
Hunziker EB, Schenk RK (1989) Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone growth in rats. J Physiol 414:55–71
Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, Richmond JC, Shelborne KD (2001) Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med 29:600–613
Kim YS, Lee HJ, Yeo JE, Kim YI, Choi YJ, Koh YG (2014) Isolation and characterization of human mesenchymal stem cells derived from synovial fluid in patients with osteochondral lesion of the talus. Am J Sports Med 43(2):399–406. doi:10.1177/0363546514559822
Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O (2007) A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am 89:2105–2112
LaPrade RF, Botker JC (2004) Donor-site morbidity after osteochondral autograft transfer procedures. Arthroscopy 20:69–73
Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S (2006) Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol 57:16–23
Niemeyer P, Salzmann G, Feucht M, Pestka J, Porichis S, Ogon P, Südkamp N, Schmal H (2014) First-generation versus second-generation autologous chondrocyte implantation for treatment of cartilage defects of the knee: a matched-pair analysis on long-term clinical outcome. Int Orthop 38:2065–2070
Oztürk A, Ozdemir MR, Ozkan Y (2006) Osteochondral autografting (mosaicplasty) in grade IV cartilage defects in the knee joint: 2- to 7-year results. Int Orthop 30:200–204
Pelissier A, Boyer P, Boussetta Y, Bierry G, Van Hille W, Hamon P, Jaeger JH, Massin P (2014) Satisfactory long-term MRI after autologous chondrocyte implantation at the knee. Knee Surg Sports Traumatol Arthrosc 22:2007–2012
Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD (1998) Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther 28:88–96
Schagemann JC, Erggelet C, Chung H-W, Lahm A, Kurz H, Mrosek EH (2009) Cell-laden and cell-free biopolymer hydrogel for the treatment of osteochondral defects in a sheep model. Tissue Eng A 15:75–82
Schneider U, Rackwitz L, Andereya S, Siebenlist S, Fensky F, Reichert J, Löer I, Barthel T, Rudert M, Nöth U (2011) A prospective multicenter study on the outcome of type I collagen hydrogel-based autologous chondrocyte implantation (CaReS) for the repair of articular cartilage defects in the knee. Am J Sports Med 39:2558–2565
Schneider U, Schmidt-Rohlfing B, Gavenis K, Maus U, Mueller-Rath R, Andereya S (2011) A comparative study of 3 different cartilage repair techniques. Knee Surg Sports Traumatol Arthrosc 19:2145–2152
Schüttler KF, Schenker H, Theisen C, Schofer MD, Getgood A, Roessler PP, Struewer J, Rominger MB, Efe T (2013) Use of cell-free collagen type I matrix implants for the treatment of small cartilage defects in the knee: clinical and magnetic resonance imaging evaluation. Knee Surg Sports Traumatol Arthrosc 22:1270–1276
Schüttler KF, Struewer J, Rominger MB, Rexin P, Efe T (2013) Repair of a chondral defect using a cell free scaffold in a young patient—a case report of successful scaffold transformation and colonisation. BMC Surg 13:11
Steadman JR, Rodkey WG, Briggs KK (2002) Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg 15:170–176
Steinwachs M (2009) New technique for cell-seeded collagen-matrix-supported autologous chondrocyte transplantation. Arthroscopy 25:208–211
Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 198:43–49
Vahdati A, Wagner DR (2013) Implant size and mechanical properties influence the failure of the adhesive bond between cartilage implants and native tissue in a finite element analysis. J Biomech 46:1554–1560
Vanlauwe J, Saris DBF, Victor J, Almqvist KF, Bellemans J, Luyten FP (2011) Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med 39:2566–2574
Wondrasch B, Risberg M-A, Zak L, Marlovits S, Aldrian S (2015) Effect of accelerated weightbearing after matrix-associated autologous chondrocyte implantation on the femoral condyle: a prospective, randomized controlled study presenting MRI-based and clinical outcomes after 5 years. Am J Sports Med 43:146–153
Zak L, Albrecht C, Wondrasch B, Widhalm H, Vekszler G, Trattnig S, Marlovits S, Aldrian S (2014) Results 2 years after matrix-associated autologous chondrocyte transplantation using the Novocart 3D scaffold: an analysis of clinical and radiological data. Am J Sports Med 42:1618–1627
Zak L, Krusche-Mandl I, Aldrian S, Trattnig S, Marlovits S (2014) Clinical and MRI evaluation of medium- to long-term results after autologous osteochondral transplantation (OCT) in the knee joint. Knee Surg Sports Traumatol Arthrosc 22:1288–1297
Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Off J Eur Union 324:121–137
Conflict of interest
Magnetic resonance imaging was supported by a research fund of Arthro Kinetics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roessler, P.P., Pfister, B., Gesslein, M. et al. Short-term follow up after implantation of a cell-free collagen type I matrix for the treatment of large cartilage defects of the knee. International Orthopaedics (SICOT) 39, 2473–2479 (2015). https://doi.org/10.1007/s00264-015-2695-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-2695-9