Abstract
Purpose
The aim of the study was to investigate the curative effects of transplantation of bone marrow mesenchymal stem cells (BMSCs) on intervertebral disc regeneration and to investigate the feasibility of the quantitative T2 mapping method for evaluating repair of the nucleus pulposus after implantation of BMSCs.
Methods
Forty-eight New Zealand white rabbits were used to establish the lumber disc degenerative model by stabbing the annulus fibrosus and then randomly divided into four groups, i.e. two weeks afterwards, BMSCs or phosphate-buffered saline (PBS) were transplanted into degenerative discs (BMSCs group and PBS group), while the operated rabbits without implantation of BMSCs or PBS served as the sham group and the rabbits without operation were used as the control group. At weeks two, six and ten after operation, the T2 values and disc height indices (DHI) were calculated by magnetic resonance imaging (MRI 3.0 T), and the gene expressions of type II collagen (COL2) and aggrecan (ACAN) in degenerative discs were evaluated by real-time reverse transcription polymerase chain reaction (RT-PCR). T2 values for the nucleus pulposus were correlated with ACAN or COL2 expression by regression analysis.
Results
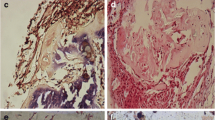
Cell clusters, disorganised fibres, interlamellar glycosaminoglycan (GAG) matrix and vascularisation were observed in lumber degenerative discs. BMSCs could be found to survive in intervertebral discs and differentiate into nucleus pulposus-like cells expressing COL2 and ACAN. The gene expression of COL2 and ACAN increased during ten weeks after transplantation as well as the T2 signal intensity and T2 value. The DHI in the BMSCs group decreased more slowly than that in PBS and sham groups. The T2 value correlated significantly with the gene expression of ACAN and COL2 in the nucleus pulposus.
Conclusions
Transplantation of BMSCs was able to promote the regeneration of degenerative discs. Quantitative and non-invasive T2 mapping could be used to evaluate the regeneration of the nucleus pulposus with good sensitivity.










Similar content being viewed by others
References
Dagenais S, Caro J, Haldeman S (2008) A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J 8:8–20
Sakai D (2008) Future perspectives of cell-based therapy for intervertebral disc disease. Eur Spine J 17(Suppl 4):452–458
Phillips FM, Reuben J, Wetzel FT (2002) Intervertebral disc degeneration adjacent to a lumbar fusion. An experimental rabbit model. J Bone Joint Surg Br 84:289–294
Rui YF, Lui PP, Lee YW, Chan KM (2012) Higher BMP receptor expression and BMP-2-induced osteogenic differentiation in tendon-derived stem cells compared with bone-marrow-derived mesenchymal stem cells. Int Orthop 36:1099–1107
Tsai MT, Lin DJ, Huang S, Lin HT, Chang WH (2012) Osteogenic differentiation is synergistically influenced by osteoinductive treatment and direct cell-cell contact between murine osteoblasts and mesenchymal stem cells. Int Orthop 36:199–205
Mueller MB, Blunk T, Appel B, Maschke A, Goepferich A, Zellner J, Englert C, Prantl L, Kujat R, Nerlich M, Angele P (2013) Insulin is essential for in vitro chondrogenesis of mesenchymal progenitor cells and influences chondrogenesis in a dose-dependent manner. Int Orthop 37:153–158
Qin L (2013) Translational medicine in orthopaedics. J Orthop Transl 1:3–5
Ciapetti G, Granchi D, Devescovi V, Leonardi E, Greggi T, Di Silvestre M, Baldini N (2012) Ex vivo observation of human intervertebral disc tissue and cells isolated from degenerated intervertebral discs. Eur Spine J 21(Suppl 1):S10–S19
Roughley P, Martens D, Rantakokko J, Alini M, Mwale F, Antoniou J (2006) The involvement of aggrecan polymorphism in degeneration of human intervertebral disc and articular cartilage. Eur Cell Mater 11:1–7, discussion 7
Longo UG, Papapietro N, Petrillo S, Franceschetti E, Maffulli N, Denaro V (2012) Mesenchymal stem cell for prevention and management of intervertebral disc degeneration. Stem Cells Int 2012:921053
Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, Nakai T, Ando K, Hotta T (2003) Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials 24:3531–3541
Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, Hotta T (2005) Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976) 30:2379–2387
Tertti M, Paajanen H, Laato M, Aho H, Komu M, Kormano M (1991) Disc degeneration in magnetic resonance imaging. A comparative biochemical, histologic, and radiologic study in cadaver spines. Spine (Phila Pa 1976) 16:629–634
Antoniou J, Pike GB, Steffen T, Baramki H, Poole AR, Aebi M, Alini M (1998) Quantitative magnetic resonance imaging in the assessment of degenerative disc disease. Magn Reson Med 40:900–907
Nightingale T, MacKay A, Pearce RH, Whittall KP, Flak B (2000) A model of unloaded human intervertebral disk based on NMR relaxation. Magn Reson Med 43:34–44
Marinelli NL, Haughton VM, Muñoz A, Anderson PA (2009) T2 relaxation times of intervertebral disc tissue correlated with water content and proteoglycan content. Spine (Phila Pa 1976) 34:520–524
Niu G, Yang J, Wang R, Dang S, Wu EX, Guo Y (2011) MR imaging assessment of lumbar intervertebral disk degeneration and age-related changes: apparent diffusion coefficient versus T2 quantitation. AJNR Am J Neuroradiol 32:1617–1623
Takashima H, Takebayashi T, Yoshimoto M, Terashima Y, Tsuda H, Ida K, Yamashita T (2012) Correlation between T2 relaxation time and intervertebral disk degeneration. Skeletal Radiol 41:163–167
Trattnig S, Stelzeneder D, Goed S, Reissegger M, Mamisch TC, Paternostro-Sluga T, Weber M, Szomolanyi P, Welsch GH (2010) Lumbar intervertebral disc abnormalities: comparison of quantitative T2 mapping with conventional MR at 3.0 T. Eur Radiol 20:2715–2722
Welsch GH, Mamisch TC, Hughes T, Zilkens C, Quirbach S, Scheffler K, Kraff O, Schweitzer ME, Szomolanyi P, Trattnig S (2008) In vivo biochemical 7.0 Tesla magnetic resonance: preliminary results of dGEMRIC, zonal T2, and T2* mapping of articular cartilage. Invest Radiol 43:619–626
Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S (2004) T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology 232:592–598
Menezes NM, Gray ML, Hartke JR, Burstein D (2004) T2 and T1rho MRI in articular cartilage systems. Magn Reson Med 51:503–509
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301
Sobajima S, Shimer AL, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, Kang JD (2005) Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J 5:14–23
Lu DS, Shono Y, Oda I, Abumi K, Kaneda K (1997) Effects of chondroitinase ABC and chymopapain on spinal motion segment biomechanics. An in vivo biomechanical, radiologic, and histologic canine study. Spine (Phila Pa 1976) 22:1828–1834, discussion 1834–1825
Longo UG, Ripalda P, Denaro V, Forriol F (2006) Morphologic comparison of cervical, thoracic, lumbar intervertebral discs of cynomolgus monkey (Macaca fascicularis). Eur Spine J 15:1845–1851
Krueger EC, Perry JO, Wu Y, Haughton VM (2007) Changes in T2 relaxation times associated with maturation of the human intervertebral disk. AJNR Am J Neuroradiol 28:1237–1241
Yang F, Leung VY, Luk KD, Chan D, Cheung KM (2009) Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol Ther 17:1959–1966
Chiu EJ, Newitt DC, Segal MR, Hu SS, Lotz JC, Majumdar S (2001) Magnetic resonance imaging measurement of relaxation and water diffusion in the human lumbar intervertebral disc under compression in vitro. Spine (Phila Pa 1976) 26:E437–E444
Xie XH, Wang XL, He YX, Liu Z, Sheng H, Zhang G, Qin L (2012) Promotion of bone repair by implantation of cryopreserved bone marrow-derived mononuclear cells in a rabbit model of steroid-associated osteonecrosis. Arthritis Rheum 64:1562–1571
Wang X, Wang Y, Gou W, Lu Q, Peng J, Lu S (2013) Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop 37:2491–2498
Watanabe A, Benneker LM, Boesch C, Watanabe T, Obata T, Anderson SE (2007) Classification of intervertebral disk degeneration with axial T2 mapping. AJR Am J Roentgenol 189:936–942
Stelzeneder D, Kovacs BK, Goed S, Welsch GH, Hirschfeld C, Paternostro-Sluga T, Friedrich KM, Mamisch TC, Trattnig S (2012) Effect of short-term unloading on T2 relaxation time in the lumbar intervertebral disc–in vivo magnetic resonance imaging study at 3.0 tesla. Spine J 12:257–264
Friedrich KM, Shepard T, de Oliveira VS, Wang L, Babb JS, Schweitzer M, Regatte R (2009) T2 measurements of cartilage in osteoarthritis patients with meniscal tears. AJR Am J Roentgenol 193:W411–415
Mosher TJ, Dardzinski BJ (2004) Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol 8:355–368
Zou J, Yang H, Miyazaki M, Morishita Y, Wei F, McGovern S, Wang JC (2009) Dynamic bulging of intervertebral discs in the degenerative lumbar spine. Spine (Phila Pa 1976) 34:2545–2550
Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA (2010) Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum 62:3695–3705
Albert HB, Sorensen JS, Christensen BS, Manniche C (2013) Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J 22:697–707
Albert HB, Lambert P, Rollason J, Sorensen JS, Worthington T, Pedersen MB, Nørgaard HS, Vernallis A, Busch F, Manniche C, Elliott T (2013) Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J 22:690–696
Aebi M (2013) Is low back pain after disc herniation with Modic type 1 changes a low-grade infection? Eur Spine J 22:689
Aebi M (2013) The two papers of Hanne Albert et al. about Modic I changes of the vertebra published in the European Spine Journal of April 2013. Editorial. Eur Spine J 22:1693
Ahmad Z, Rai A, Donell S, Crawford R (2013) Letter to the editor concerning: “Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized controlled trial of efficacy” by Albert HB et al. Eur Spine J (2013) 22:697–707. Eur Spine J 22:2344–2345
Almefty KK, Turner JD, Theodore N (2013) From narcotics to antibiotics: evolving concepts in the treatment of lower back pain. World Neurosurg 80:442–443
Dean BJ (2013) Do these results apply to the ‘intervention naive’ patient? Eur Spine J 22:1702
Sotto A, Dupeyron A (2013) Letter to the editor concerning: “Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized controlled trial of efficacy” by Albert HB et al. Eur Spine J 22:697–707. Eur Spine J 22:1704–1705
O’Dowd J, Casey A (2013) Antibiotics a cure for back pain, a false dawn or a new era? Eur Spine J 22:1694–1697
Shubhakaran K, Khichar RJ (2013) Backache and infection. Eur Spine J 22:2348
Lings S (2014) Antibiotics for low back pain? Eur Spine J 23:469–472
Acknowledgments
The work was supported by the National Natural Science Foundation of China (81272035; 81071493; 81201423) and the 51st China Postdoctoral Science Foundation 2012 (2012 M511800). Thanks to Prof. Ling Qin (The Chinese University of Hong Kong) for language editing. Thanks to Yuan-cheng Wang (Southeast University) for MRI evaluation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cai, F., Wu, XT., Xie, XH. et al. Evaluation of intervertebral disc regeneration with implantation of bone marrow mesenchymal stem cells (BMSCs) using quantitative T2 mapping: a study in rabbits. International Orthopaedics (SICOT) 39, 149–159 (2015). https://doi.org/10.1007/s00264-014-2481-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2481-0