Abstract
Purpose
Nitric oxide (NO) synthesised by endothelial NO synthase (eNOS) is a potent regulator of internal haemodynamics. A polymorphism in intron 4 of the eNOS is associated with different vascular disorders. We investigated the potential involvement of this polymorphism in idiopathic and secondary osteonecrosis of the femoral head (ONFH) in Polish patients.
Methods
We performed a study involving 68 patients with ONFH (45 idiopathic and 23 secondary) and 100 healthy controls. All subjects were genotyped for the eNOS4 polymorphism by the polymerase chain reaction followed by agarose gel electrophoresis.
Results
The analysis revealed that the frequencies of eNOS4 genotypes were significantly different in ONFH patients (both idiopathic and secondary) than in controls. The frequencies of the 4a allele were significantly higher in the total group of patients versus controls [22.79 vs 9 %, p = 0.00039, odds ratio (OR) 2.98]. In subgroup analysis the 4a allele increased significantly in both idiopathic (20 vs 9 %, p = 0.0074, OR = 2.52) and secondary (28.26 vs 9 %, p = 0.00047, OR = 3.98) ONFH patients compared to control subjects. The frequency of the 4a/b genotype in the total group of patients (36.76 vs 16 %, p = 0.0011, OR = 3.24) as well as patients with idiopathic (35.56 vs 16 %, p = 0.0069, OR = 2.96) and secondary (39.13 vs 16 %, p = 0.0073, OR = 3.89) ONFH was higher than in the control group.
Conclusions
There was a significantly higher frequency of eNOS 4a allele carriers among the total group of patients as well as in idiopathic and secondary ONFH. This suggests that the eNOS gene polymorphism may be associated with increased risk of ONFH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of osteonecrosis of the femoral head (ONFH) is not well known. It is assumed that from 10,000 to 20,000 new cases of ONFH are annually identified in the USA [24]. ONFH occurs mainly in young and active patients, usually between 30 and 50 years old. Osteonecrosis of the femoral head accounts for 10 % of total hip arthroplasties performed every year in the USA and Western Europe [24].
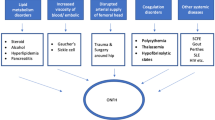
ONFH is a disease with multiple aetiologies with the death of osteocytes as the end stage of different pathological processes [2]. The most common causes of secondary ONFH are steroids, alcohol abuse, haemoglobinopathies, hyperlipidaemia, storage diseases, pregnancy, hyperbarism, solid organs and bone marrow transplantations, and chemotherapy [2].
One of the possible causes of ONFH is occlusion of vessels responsible for blood supply of the femoral head because of thrombophilia and/or hypofibrinolysis [13]. Several investigations have been performed to reveal the genetic background of thrombophilia-related ONFH [9]. Recently the relationship between endothelial nitric oxide synthase (eNOS) polymorphisms and ONFH has been reported [10, 14]. Nitric oxide (NO) is an intracellular messenger which plays an important role in homoeostasis, vascular system and bone turnover [21]. Nitric oxide inhibits platelet activation, adhesion and aggregation [19]. It was also revealed that NO is a vasodilatation molecule, and NO deficiency causes vasospasm [5]. Impairment in NO production diminishes angiogenesis [18]. Nitric oxide controls microvascular permeability [6]. It was reported that NO regulates bone mass and bone turnover through effects on osteoclast and osteoblast activity [20] and inhibits osteoclasis [15].
Nitric oxide is synthesised from L-arginine by three isoforms of synthases (NOS): neuronal (nNOS), inducible (iNOS) and endothelial (eNOS). Nitric oxide synthase isoforms share 50 % homology and are encoded by different genes [17]. Endothelial nitric oxide synthase is predominantly a constitutive isoform expressed in normal adult bone [8]. Human eNOS is encoded by a gene located on chromosome 7q35-36 comprising 26 exons and 25 introns and its predominant form has 133 kDa [1]. The enzymatic activity of the constitutively expressed eNOS is controlled by intracellular Ca2+ levels and other cofactors. After being released in endothelial cells, NO diffuses rapidly through cell membranes and relaxes neighbouring vascular smooth muscle cells through the production of guanosine 3′,5′-cyclic monophosphate (cGMP).
Over the last few years several polymorphisms of the eNOS gene have been identified, and their association with various diseases has been explored [25]. Most of the attention has focused on a single nucleotide polymorphism in the promoter region (T-786-C), a single nucleotide polymorphism in exon 7 (Glu296Asp) and a variable number of tandem repeats in intron 4. In the 27-bp repeat in intron 4, two alleles have been identified, the larger of which, eNOS4b, has five tandem 27-bp repeats [GAAGTCTAGACCTGCTGC(A/G)GGGGTGAG] and the smaller, eNOS4a, has four repeats. It was revealed that NO levels were significantly lower in the subjects with the 4a allele than in those without the presence of the 4a allele [23].
Nitric oxide plays a role in bone angiogenesis, thrombosis and turnover, all of which are probably related to the pathogenesis of osteonecrosis [4]. The aim of this study was to investigate possible correlations between the eNOS intron 4 polymorphism and non-traumatic ONFH in Polish patients.
Subjects and methods
Patients
A group of 68 unrelated adults (106 hips) were involved in this study. All patients were Caucasians of Polish origin coming from the eastern part of Poland. Patients were admitted to the Orthopaedic and Traumatology Department for operative treatment of ONFH. ONFH was diagnosed by clinical examination and radiographic analysis. Patients without characteristic risk factors related to ON were classified as having idiopathic ONFH (n = 45). The cause of ONFH was established in 23 patients: the use of corticosteroids (n = 11), chemotherapy (n = 7), alcoholism (n = 4) and renal transplantation (n = 1). These patients were classified as having secondary ONFH. Patients with idiopathic and secondary ONFH did not differ with respect to average age and gender distribution. The Ficat and Arlet classification was used for radiographic evaluation [7]. Anteroposterior (AP) and frog view X-rays of both hips were done in all of the patients. Magnetic resonance imaging (MRI) was performed to confirm the diagnosis of ONFH in patients without X-ray changes (Figs. 1a, b and 2). ONFH was present in one hip in 30 patients and in both hips in 38 patients (106 hips). Stage I of ONFH according to Ficat and Arlet [7] was seen in 14 hips, II in 19 hips, III in 22 hips and IV in 51 hips. Healthy control subjects (n = 100) with no clinical signs and family history of ONFH were recruited among blood donors and hospital staff. All participants in the control group also came from the eastern part of Poland.
A written informed consent for genetic studies was obtained from all patients and members of the control group. The study protocol was evaluated and approved by the Ethics Committee at the Medical University in Lublin.
Determination of eNOS genotype
Genomic DNA was isolated from peripheral blood leucocytes using the method described by Madisen et al. [16] with minor modifications. For polymerase chain reaction (PCR) amplification, two oligonucleotide primers were used that flank the region of the 27-bp repeat sequence in intron 4 of the eNOS gene. The forward primer was 5′-AGGCCCTATGGTAGTGCCTTT-3′ and the reverse primer was 5′-tctcttagtgctgtggtcac-3′. Genomic DNA (300 ng) was amplified in a final reaction volume of 50 μl, containing 10 mM Tris pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 200 μM each dNTP, 1 μM of each primer and 2 U Taq polymerase (all reagents from MBI Fermentas). The reaction mixture was heated to 94 °C for six minutes for denaturation and then subjected to 35 cycles at 94 °C for one minute, annealing at 56 °C for one minute and extension at 72 °C for two minutes. Final extension was at 72 °C for seven minutes. The PCR products were analysed by electrophoresis in 2.5 % agarose gels stained with ethidium bromide (Fig. 3).
Statistical analysis
All calculations were performed using SPSS for Windows 17.0. Normally distributed data are presented as means ± SD. Genotype distribution and allele frequencies were compared between groups using the χ2 test and Fisher’s exact test, respectively. Odds ratios (ORs) and 95 % confidence intervals (CIs) were calculated using relevant 2 × 2 contingency tables.
Results
We genotyped the eNOS intron 4 polymorphism in 68 patients with ONFH and 100 healthy controls. Clinical characteristics of the study group are summarised in Table 1. No significant differences between groups with respect to age and gender ratio were observed. Genotype and allele frequencies of ONFH risk patients are showed in Tables 2 and 3. The genotype frequencies of the analysed polymorphism were in Hardy-Weinberg equilibrium. All alleles and genotypes were observed in the ONFH and control groups, even the rare 4a/a genotype. When compared to the control group the frequency of the 4a allele was higher in the total group of patients (22.79 vs 9 %, p = 0.00039, OR = 2.98, 95 % CI 1.59–5,59), idiopathic osteonecrosis (20 vs 9 %, p = 0.0074, OR = 2.52, 95 % CI 1.24–5.13) and secondary osteonecrosis subgroups (28.26 vs 9 %, p = 0.00047, OR = 3.98, 95 % CI 1.78–8.9). The frequency of the 4a/a genotype was significantly higher in the secondary ONFH subgroup than in controls (8.7 vs 1 %, p = 0.036, OR = 13.83, 95 % CI 1.16–164.4). The 4a/a genotype frequency tended to be higher in other groups than in controls but was not statistically significant. The frequency of the 4a/b genotype was statistically significant in patients (36.76 vs 16 %, p = 0.0011, OR = 3.24, 95 % CI 1.55–6.74), idiopathic (35.56 vs 16 %, p = 0.0069, OR = 2.96, 95 % CI 1.31–6.69) and secondary (39.13 vs 16 %, p = 0.0073, OR = 3.89, 95 % CI 1.4–10.75) subgroups.
Discussion
Koo et al. [14] were the first to report on the relationship between eNOS polymorphisms and ONFH. The study was performed in Korean patients. They found significantly higher frequencies of the 4a allele in the total group of patients (6.8 vs 2.4 %, p = 0.0345, OR = 2.931) and idiopathic ONFH subgroup (9.0 vs 2.4 %, p = 0.0297, OR = 3.976) than in the control group. The rare 4a/a genotype was not found in patients with ONFH. The frequency of the the 4a/b genotype in the total group of patients (13.6 vs 4.9 %, p = 0.0302, OR = 3.083) as well as in patients with idiopathic ONFH (18.0 vs 4.9 %, p = 0.0246, OR = 4.302) was higher than in control subjects. The frequency of the intron 4 polymorphism did not differ when patients with ONFH secondary to steroids or alcohol abuse were compared with controls. No other reports on a relationship between the eNOS 4 intron polymorphism and ONFH have as yet been published. Our study presents the results in Caucasian patients and a control group coming from one geographical region. We found higher frequencies of the 4a allele and 4a/b genotype in both the total group of patients and the idiopathic ONFH group, but also in the secondary ONFH subgroup. The rare 4a/a genotype was present in our study and was statistically significantly more frequent in the secondary ONFH subgroup. The differences in results show the impact of ethnicity on the prevalence of the mutations [22]. Our results show a strong association between the eNOS polymorphism and ONFH. Our results support the conclusions of Koo et al. [14] that since the 4a polymorphism is associated with reduced synthesis of eNOS a carrier state of the 4a allele in intron 4 might be a genetic risk factor for ONFH and could provide insight into the protective role of NO in the pathogenesis of this condition.
Glueck et al. [10] reported an association between the T-786-C eNOS polymorphism and idiopathic ONFH. Patients differed in race: white 85 %, black 9 % and other 4 %. The authors found a higher TT mutant allele frequency in the idiopathic osteonecrosis group than in the race-, gender- and age-matched control group (22 vs 3 %, OR = 6.0, 95 % CI 2.51–14.4). In addition to this they did not find an association of the T-786-C eNOS polymorphism and allele frequency between secondary ONFH and the race-, gender- and age-matched control group. Patients with primary ONFH had a higher frequency of mutant genotype and mutant allele frequency than the secondary ONFH group. In comparison to the papers of Koo et al. [14] and Glueck et al. [10], we found a positive correlation between higher frequency of eNOS polymorphism and allele frequency in the secondary ONFH group as well as in the idiopathic ONFH group. An impairment of NO production may play a synergistic role with other conditions in the development of secondary ONFH. Our results suggest that the eNOS polymorphism may contribute to the pathogenesis of both idiopathic and secondary ONFH. Later studies revealed a statistically significant correlation between the T-786-C eNOS polymorphism and idiopathic as well as secondary multifocal osteonecrosis [11]. The T-786-C eNOS polymorphism was reported as a potential cause of neuralgia-inducing cavitational osteonecrosis of the jaws [12].
There were reports suggesting that smoking decreases eNOS activity and may modify relations between polymorphisms and expression levels [3]. Both Koo et al. [14] and Glueck et al. [10] revealed that smoking is a synergistic risk factor for ONFH in association with the eNOS polymorphism in idiopathic ONFH. We observed more smokers in the idiopathic ONFH group than in the secondary group, but the relationship between smoking and the eNOS polymorphism was not studied because data about smoking in the control group was lacking.
In conclusion, the eNOS 4a gene polymorphism was associated with ONFH in Polish patients. Reduction in NO production causes promotion of platelet recruitment, aggregation and adhesion, impairing angiogenesis and reducing bone formation. The eNOS polymorphism demonstrates a multidirectional cause of ONFH via the eNOS polymorphism. The eNOS polymorphism may be an independent cause of idiopathic osteonecrosis and may be a synergistic cause of secondary ONFH with other patho-aetiologies of osteonecrosis. The strength of this study lies in being the first to examine the association between the eNOS polymorphism and ONFH in ethnically homogeneous Caucasians. Our study has some limitations. The small sample size and investigation of unselected cases may influence the results. Comparisons with other eNOS polymorphisms were not examined. Despite these limitations, the association of the eNOS gene intron 4 polymorphism with ONFH susceptibility will strengthen our understanding of the relationship between eNOS dysfunction and ONFH pathogenesis.
References
Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357:593–615
Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME (2002) Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum 32:94–124
Barua RS, Ambrose JA, Eales-Reynolds LJ, DeVoe MC, Zervas JG, Saha DC (2001) Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation 104:1905–1910
Calder JD, Buttery L, Revell PA, Pearse M, Polak JM (2004) Apoptosis—a significant cause of bone cell death in osteonecrosis of the femoral head. J Bone Joint Surg Br 86:1209–1213
Cooke JP (2004) The pivotal role of nitric oxide for vascular health. Can J Cardiol 20(Suppl B):7B–15B
Dusserre N, L’Heureux N, Bell KS, Stevens HY, Yeh J, Otte LA, Loufrani L, Frangos JA (2004) PECAM-1 interacts with nitric oxide synthase in human endothelial cells: implication for flow-induced nitric oxide synthase activation. Arterioscler Thromb Vasc Biol 24:1796–1802
Ficat RP, Arlet J (1984) Necrosis of the femoral head. In: Hungerford DS (ed) Ischemia and necrosis of bone. Williams & Wilkins, Baltimore, pp 53–74
Fox SW, Chow JW (1998) Nitric oxide synthase expression in bone cells. Bone 23:1–6
Glueck CJ, Freiberg RA, Fontaine RN, Tracy T, Wang P (2001) Hypofibrinolysis, thrombophilia, osteonecrosis. Clin Orthop Relat Res 386:19–33
Glueck CJ, Freiberg RA, Oghene J, Fontaine RN, Wang P (2007) Association between the T-786C eNOS polymorphism and idiopathic osteonecrosis of the head of the femur. J Bone Joint Surg Am 89:2460–2468
Glueck CJ, Freiberg RA, Boppana S, Wang P (2008) Thrombophilia, hypofibrinolysis, the eNOS T-786C polymorphism, and multifocal osteonecrosis. J Bone Joint Surg Am 90:2220–2229
Glueck CJ, McMahon RE, Bouquot JE, Khan NA, Wang P (2010) T-786C polymorphism of the endothelial nitric oxide synthase gene and neuralgia-inducing cavitational osteonecrosis of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:548–553
Jones JP Jr (1992) Intravascular coagulation and osteonecrosis. Clin Orthop Relat Res 277:41–53
Koo KH, Lee JS, Lee YJ, Kim KJ, Yoo JJ, Kim HJ (2006) Endothelial nitric oxide synthase gene polymorphisms in patients with nontraumatic femoral head osteonecrosis. J Orthop Res 24(8):1722–1728
Loveridge N, Fletcher S, Power J, Caballero-Alías AM, Das-Gupta V, Rushton N, Parker M, Reeve J, Pitsillides AA (2002) Patterns of osteocytic endothelial nitric oxide synthase expression in the femoral neck cortex: differences between cases of intracapsular hip fracture and controls. Bone 30:866–871
Madisen L, Hoar LD, Holroyd CD, Crisp M, Hodes ME (1987) DNA banking: the effect of storage of blood and isolated DNA on the integrity of DNA. Am J Med Genet 27:379–390
Nadaud S, Bonnardeaux A, Lathrop M, Soubrier F (1994) Gene structure, polymorphism and mapping of the human endothelial nitric oxide synthase gene. Biochem Biophys Res Commun 198:1027–1033
Namba T, Koike H, Murakami K, Aoki M, Makino H, Hashiya N, Ogihara T, Kaneda Y, Kohno M, Morishita R (2003) Angiogenesis induced by endothelial nitric oxide synthase gene through vascular endothelial growth factor expression in a rat hindlimb ischemia model. Circulation 108:2250–2257
Radomski MW, Palmer RM, Moncada S (1987) Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 2:1057–1058
Samuels A, Perry MJ, Gibson RL, Colley S, Tobias JH (2001) Role of endothelial nitric oxide synthase in estrogen-induced osteogenesis. Bone 29:24–29
Schmidt HH, Walter U (1994) NO at work. Cell 78:919–925
Tanus-Santos JE, Desai M, Flockhart DA (2001) Effects of ethnicity on the distribution of clinically relevant endothelial nitric oxide variants. Pharmacogenetics 11:719–725
Tsukada T, Yokoyama K, Arai T, Takemoto F, Hara S, Yamada A, Kawaguchi Y, Hosoya T, Igari J (1998) Evidence of association of the ecNOS gene polymorphism with plasma NO metabolite levels in humans. Biochem Biophys Res Commun 245:190–193
Vail TP, Covington DB (1997) The incidence of osteonecrosis. In: Urbaniak JR, Jones JP Jr (eds) Osteonecrosis: etiology, diagnosis, and treatment. American Orthopaedic Association, Rosemont, pp 43–49
Wang XL, Wang J (2000) Endothelial nitric oxide synthase gene sequence variations and vascular disease. Mol Genet Metab 70:241–251
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Gagala, J., Buraczynska, M., Mazurkiewicz, T. et al. Endothelial nitric oxide synthase gene intron 4 polymorphism in non-traumatic osteonecrosis of the femoral head. International Orthopaedics (SICOT) 37, 1381–1385 (2013). https://doi.org/10.1007/s00264-013-1892-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-013-1892-7