Abstract
Objectives
To investigate the feasibility and potential clinical value of local consolidative therapy (LCT) in PD-1/PD-L1 inhibitor-treated metastatic non-small cell lung cancer (NSCLC).
Materials and methods
PD-1/PD-L1 inhibitor-treated metastatic NSCLC patients with measurable disease in three academic centers were screened and those with adequate follow-up were included. Oligo-residual disease (ORD) was defined as residual tumors limited to three organs and five lesions evaluated at the best response among patients with partial response or stable disease after PD-1/PD-L1 inhibitors. Oligometastatic and multiple-metastatic disease (OMD/MMD) were similarly classified at baseline. Locoregional interventions, administered after effective treatment of PD-1/PD-L1 inhibitors and before initial disease progression, were defined as LCT. Patterns of initial progressive disease (PD) were classified as involving only residual sites (RP), only new sites (NP), or a combination of both (BP).
Results
Among the 698 patients included, ORD was documented in 73 (47.1%) of 155 patients with baseline OMD and 60 (11.0%) of 543 patients with baseline MMD. With a median follow-up of 31.0 (range, 6.0–53.0) months, 108 patients with ORD developed initial PD, with RP, NP, and BP occurring in 51 (47%), 23 (21.3%), and 34 (31.5%), respectively. Among the 133 patients with ORD, those receiving LCT (n = 43) had longer progression-free survival (HR = 0.58, 95% CI 0.40–0.85, p = 0.01) and overall survival (HR = 0.49, 95% CI 0.30–0.79, p < 0.0001).
Conclusion
ORD occurs with a clinically relevant frequency among PD-1/PD-L1 inhibitor-treated metastatic NSCLC patients and LCT may provide extra survival benefits in those with ORD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The advent of immunotherapy has revolutionized the management of metastatic non-small cell lung cancer (NSCLC). PD-1/PD-L1 inhibitor monotherapy or its combination with chemotherapy is now established as the first-line standard of care for metastatic NSCLC patients lacking driver mutations [1,2,3]. However, only a subset of patients benefit from immunotherapy [4], and many initially responsive patients eventually develop acquired resistance due to complex mechanisms [5]. This situation complicates the achievement of a universally effective salvage systemic therapy, prompting the exploration of combination strategies to mitigate and counteract resistance in patients undergoing immunotherapy [6].
The concept of oligo-residual disease (ORD), inspired by the oligo-metastasis paradigm initially described in 1995 [7], has garnered increasing attention in recent years [8,9,10]. Local consolidative therapy (LCT), particularly radiotherapy, has been demonstrated to enhance survival in advanced NSCLC patients with ORD following effective systemic treatment [9, 10]. At maximal response to systemic therapy, patients typically present with the lowest tumor burden, making local interventions targeting residual sites most likely to yield substantial efficacy with minimal side effects [11, 12]. Additionally, drug-resistant clones within residual tumor lesions may act as seeds for future metastases; thus, encompassing LCT may delay drug resistance onset or even extend survival [13, 14]. In the realm of targeted therapy, our prior research indicated that over one-fourth of patients with epidermal growth factor receptor (EGFR)-mutant metastatic NSCLC receiving third-generation EGFR tyrosine kinase inhibitors (TKIs) exhibited ORD at the peak response to EGFR-TKIs [10]. Patients receiving LCT in real-world settings showed notably longer survival [15], findings partly corroborated by the prospective ATOM study [16]. However, the exploration of LCT’s clinical value in immunotherapy-treated metastatic NSCLC with ORD remains unreported. Notably, radiotherapy-induced cell death can trigger immunogenic effects, potentially synergizing with immunotherapy [17,18,19]. Consequently, radiotherapy-based LCT might play pivotal roles, such as enhancing systemic anti-tumor immune responses and fostering long-term survival, in patients treated with PD-1/PD-L1 inhibitors for metastatic NSCLC with ORD, meriting further examination. Nonetheless, the prevalence of ORD in patients undergoing PD-1/PD-L1 inhibitor therapy and the clinical applicability of LCT in such cases remain largely unexplored.
This multicenter retrospective study extensively analyzes tumor regression patterns at the optimal response to PD-1/PD-L1 inhibitors and instances of treatment failure in patients with ORD. Additionally, it investigates the clinical significance of LCT in patients exhibiting ORD.
Materials and methods
Patient
Patients with metastatic NSCLC treated with PD-1/PD-L1 inhibitors, either as monotherapy or in combination with other drugs, between May 2018 and December 2021 at three institutions (Fudan University Shanghai Cancer Center, Fudan University Zhongshan Hospital, and Tongji Hospital affiliated with Tongji Medical College of Huazhong University of Science and Technology) were retrospectively reviewed, as previously described [20]. Eligible patients included those with at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors guideline (version 1.1). Exclusion criteria encompassed patients who received concurrent local therapy within four weeks of initiating PD-1/PD-L1 therapy. Neoplasm staging adhered to the eighth edition of the American Joint Committee on Cancer staging manual. The study received ethical approval from the institutional review boards of all three medical centers (Approval number: 2012228-3).
LCT, encompassing surgery, radiotherapy, and radiofrequency ablation, targeted residual tumor lesions post-effective PD-1/PD-L1 inhibitor treatment and prior to initial disease progression in a subset of patients (the LCT group). In contrast, the non-LCT group consisted of patients receiving only anti-PD-1/PD-L1 therapy without any loco-regional intervention before disease progression. For patients with stable ORD, systemic therapy continued until disease progression or the emergence of unacceptable toxicities. The decision to administer LCT was at the discretion of the treating physicians, and pertinent data were retrospectively collected for this study.
Follow-up and response assessment
Baseline imaging for all patients prior to initiating immunotherapy included enhanced computed tomography (CT) of the chest, enhanced magnetic resonance imaging (MRI) or CT of the head, enhanced CT or ultrasound of the abdomen, and a whole-body bone scan, or whole-body positron emission tomography (PET)/CT plus enhanced MRI or CT of the head. The patients were typically followed up every 2–3 months in accordance with the institution’s standard of care and relevant guidelines. During each follow-up, comprehensive radiographic assessments were conducted, encompassing chest CT scans, CT scans or ultrasonography of the abdominal and cervical regions. Meanwhile, for those with baseline brain metastasis, brain MRI or CT was performed at each radiographic follow-up. For those with baseline bone metastasis, whole-body bone scan was generally performed every 6 months. Nevertheless, PET/CT was not mandatory and performed at the treating physicians’ discretion. All serial imaging for each patient was meticulously reviewed by a senior radiologist. Telephone follow-ups were conducted as needed. The data cut-off date was 31 December 2023.
Definition of ORD and pattern of failure analyzes
The imaging scans of patients who achieved a partial response (PR) or stable disease (SD) as their best response to anti-PD-1/PD-L1 therapy were meticulously analyzed. Those with residual tumor distribution confined to three organs and a maximum of five lesions amenable to radical local treatment were categorized as having ORD [10, 15, 21]. Baseline oligometastatic disease (OMD) was characterized by ≤ 5 metastases and involvement of ≤ 3 organs, where all metastatic sites, including primary tumors and lymph nodes, were amenable to radical local treatment [7, 21]; cases not meeting these criteria were classified as baseline multiple metastatic disease (MMD).
Patterns of initial progressive disease (PD) were stratified into three types based on the relationship between progressive and residual tumor lesions. Residual lesion progression (RP) was identified when disease progression occurred exclusively at residual lesions. New lesion progression (NP) referred to the emergence of new tumors that were not present at the time of the best response to anti-PD-1/PD-L1 therapy. Cases where initial disease progression involved both residual and new lesions were defined as both lesion progression (BP).
Statistical analyzes
Baseline characteristics of the study were summarized using descriptive statistics and compared via chi-square contingency analyzes. Progression-free survival (PFS) was defined as the duration from the commencement of immunotherapy to the occurrence of disease progression or death due to any cause. Overall survival (OS) was calculated from the start of immunotherapy to death from any cause. The Kaplan–Meier method, accompanied by the log-rank test, was employed to assess PFS and OS. To evaluate the impact of LCT on PFS and OS in patients with ORD, Cox proportional hazard models were used within various subgroups. Adverse events (AEs) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.A p value of < 0.05 was considered statistically significant. All analyzes were performed using the Statistical Package for the Social Sciences (SPSS) software (version 20.0, SPSS Inc., Chicago, IL, USA) and GraphPad Prism (version 7.00) for Windows (GraphPad Software).
Results
Patient characteristics
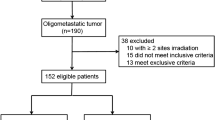
Between May 2018 and December 2021, a total of 926 patients with metastatic NSCLC undergoing PD-1/PD-L1 inhibitor treatment across three academic centers were retrospectively reviewed. Out of these, 698 patients were ultimately included in the study (as shown in Fig. 1). Among the included patients, OMD and MMD were identified in 155 (22.2%) and 543 (77.8%) patients, respectively. Comprehensive demographic data and baseline disease characteristics for these 698 patients are detailed in Table 1.
Clinical features of ORD
During a median follow-up period of 31.0 months (range, 6.0–53.0 months), partial response (PR), stable disease (SD), and PD were observed as the best responses in 265 (38.0%), 258 (37.0%), and 175 (25.1%) patients, respectively. Notably, 73 (47.1%) of the 155 patients with baseline OMD and 60 (11.0%) of the 543 patients with baseline MMD developed ORD, as depicted in Fig. 1. Detailed demographics and baseline disease characteristics for the 133 ORD patients are provided in Supplementary Table 1. Among them, 47 (35.3%) patients underwent monotherapy with PD-1/PD-L1 inhibitors, while the remaining 86 (64.7%) received combination therapy. Additionally, PD-1/PD-L1 inhibitors were utilized as first-line therapy in 52 (39.1%) patients and as second-line or subsequent therapy in 81 (60.9%) patients, as shown in Supplementary Fig. 1. Cox regression analyzes were conducted to identify predictors of ORD. Univariate analysis indicated significant associations between treatment lines (first-line vs. second or subsequent line) and baseline disease states (OMD vs. MMD) with the development of ORD (p ≤ 0.001, Table 2). Multivariate analysis further identified first-line immunotherapy and baseline OMD as independent predictors of ORD (Table 2).
Among the ORD patients, the median time to maximal response was 4.0 months (range 1.0–27.0 months). Regarding residual organ involvement, 50 (37.6%) patients had involvement in a single organ, while 83 (62.4%) had 2–3 involved organs, as illustrated in Supplementary Fig. 2A. In terms of residual tumor lesions, 36 (27.1%) patients had a single lesion, and 97 (72.9%) had 2–5 lesions, as demonstrated in Supplementary Fig. 2B. The lungs were the most common site of residual disease, detailed in Supplementary Fig. 2C and D.
Pattern of treatment failure among patients with ORD
Of the 133 patients diagnosed with oligo-residual disease (ORD), 43 (32.3%) underwent one or more forms of LCT, which included radiotherapy (39 patients), hepatic radiofrequency (3 patients), and surgery (3 patients), as detailed in Table 3. Notably, 24 of these patients received either stereotactic or hypo-fractionated radiotherapy. The baseline characteristics were largely comparable between the LCT and non-LCT groups, except for a higher prevalence of smoking history in the LCT group (58.1% vs. 35.6%, as shown in Supplementary Table 1).
By the time of data cut-off, 108 (81.2%) of the 133 ORD patients experienced initial PD. In fact, 74 (68.5%) of the 90 patients in the non-LCT group and 34 (31.5%) of the 43 patients in the LCT group developed their initial PD. Specifically, RP occurred in 51 (47.0%) patients, NP in 23 (21.3%), and BP in 34 (31.5%). In comparison, 56 (62.2%) of the 90 patients in the non-LCT group developed initial PD. The distribution of RP, NP, and BP between the LCT and non-LCT groups was not significantly different (p = 0.122), as depicted in Fig. 2.
Patterns of disease progression in patients with oligo-residual disease. Venn diagram of patterns of disease progression in LCT group (upper) and non-LCT group (lower) of patients with ORD at maximal response. LCT: local consolidation treatments, ORD: oligo-residual disease, RP: residual lesion progression, NP: new lesion progression, BP: both lesion progression
Clinical outcomes among patients with ORD
Among the 133 patients with ORD, the median PFS was 12.0 (95% CI 9.09–12.99) months. Of them, the median PFS was 18.0 (95% CI 9.87–26.13) and 10.0 (95% CI 8.73–12.07) months among those with and without LCT, respectively. Patients who underwent LCT experienced a significantly longer PFS (p = 0.01, Hazard Ratio [HR] = 0.58, 95% CI: 0.40–0.85), when compared to those who did not receive LCT (Fig. 3A).
By the time of data cut-off, 67 (50.4%) patients had died and the median OS was 40.0 (95% CI 34.61–45.39) months in the entire cohort of patients with ORD. Of them, the median OS was 44.0 (95% CI 39.78–48.22) and 30.0 (95% CI 20.67–40.53) months among those with and without LCT, respectively. Notably, LCT was associated with a significant extension in OS (HR = 0.49, 95% CI 0.30–0.79, p < 0.0001), depicted in Fig. 3B. Furthermore, LCT was identified as an independent predictor of improved PFS and OS following Cox regression analyzes, as detailed in Supplementary Tables 2 and 3.
Meanwhile, grade 3–5 treatment-related AEs among the 133 patients with ORD were summarized in supplemental Table 4. The incidence of grade 3–5 treatment-related AEs were similar among those with or without LCT.
Discussion
Local consolidative therapy is gaining traction among metastatic non-small cell lung cancer (NSCLC) patients with ORD following effective systemic therapy. Previous studies, including our own, have provided critical evidence supporting the survival benefits of LCT in the context of chemotherapy and targeted therapy [1,2,3, 10, 15]. Given the potential synergistic effects between radiotherapy and immunotherapy, the role of consolidative radiotherapy in immunotherapy-treated patients with ORD demands thorough investigation. To our knowledge, this study is the first to examine the clinical characteristics of ORD and assess the clinical value of LCT in metastatic NSCLC patients treated with PD-1/PD-L1 inhibitors, offering preliminary evidence for the potential clinical significance of consolidative radiotherapy in this patient group.
The clinical occurrence of ORD is notable in metastatic NSCLC patients treated with EGFR tyrosine kinase inhibitors (EGFR–TKIs). Taichi Miyawaki’s analysis of 207 patients with advanced EGFR-mutated NSCLC treated with first-line EGFR–TKIs over seven years revealed that 32% experienced ORD [22]. Similarly, our previous study found that 26.8% of patients with metastatic NSCLC treated with Osimertinib developed ORD at maximal response, suggesting their suitability for consolidative stereotactic radiotherapy [10]. However, this study marks the first instance of determining ORD frequency (19.1%) in the era of immunotherapy using real-world data from a sizeable sample, a finding that requires validation in prospective trials.
In targeted therapies for NSCLC, about half of the patients experience initial progressive disease (PD) exclusively from residual tumor lesions [10], a finding mirrored in our study among patients receiving immunotherapy. Notably, substantial intra-tumor and inter-tumor heterogeneities, particularly in genetic aberrations and immune microenvironments, are prevalent in metastatic NSCLC [23,24,25,26]. These heterogeneities can result in varying drug sensitivities across tumor lesions, with drug-resistant sub clones potentially becoming the source of residual disease and, ultimately, the seed for PD. Accumulating evidence indicates that most patients who acquire resistance to PD-1/PD-L1 inhibitors develop oligo-progressive disease originating from pre-existing tumor sites [27]. Theoretically, preemptive local therapy aimed at eradicating these residual lesions could prevent the systemic spread of resistant clones. Additionally, local consolidative therapy (LCT) plays a critical role in preventing local symptoms and complications associated with tumor growth. Therefore, local interventions targeting residual lesions prior to disease progression are potentially valuable in managing metastatic NSCLC after effective systemic treatment.
LCT, particularly beneficial in patients under conventional therapy [9, 15, 28, 29], is now, for the first time in this study, also shown to offer additional survival benefits for metastatic NSCLC with ORD in the context of immunotherapy. As a primary local treatment, radiotherapy, when combined with immunotherapy, can significantly enhance the antitumor immune response, leading to improved tumor control [30]. For instance, radiotherapy not only increases tumor cell susceptibility to T-cell-mediated attacks but also induces immunogenic cell death. This process involves promoting the release of tumor antigens from dead cells, enhancing MHC class I expression, and upregulating immune regulatory cell surface molecules [31]. Furthermore, radiotherapy upregulates PD-L1 expression to enhance radio-sensitization and counteract adaptive immune resistance [32, 33]. The integration of radiotherapy with immunotherapy in NSCLC patients has thus garnered extensive interest [34]. Additionally, in immunotherapy, a lower tumor burden correlates with higher treatment efficacy in lung cancer and other solid tumors [35, 36]. Consequently, effective debunking by LCT may help eliminate drug-resistant tumor clones and bolster systemic antitumor immune responses in patients with ORD. In our study, LCT was demonstrated to extend both progression-free survival (PFS) and overall survival (OS) using real-world data, underscoring the need for further validation in future research.
Compared to salvage local therapy in patients with oligo-progressive disease [37,38,39,40], local consolidative therapy (LCT) in those with oligo-residual disease (ORD) may offer several advantages. Our recent study in patients with EGFR-mutant advanced NSCLC receiving Osimertinib showed that, compared to salvage radiotherapy in patients with oligo-progressive disease, consolidative radiotherapy targeting EGFR-TKI resistant clones at all oligo-residual tumor sites potentially offered a survival advantage [10]. Similarly, in the context of immunotherapy, consolidative local therapy may have benefits over salvage local therapy. Firstly, tumor burden is at its lowest following maximal response to PD-1/PD-L1 inhibitors, making consolidative local therapy at this juncture potentially more effective and less toxic [11, 12]. Secondly, while the majority of immunotherapy patients develop oligo-progressive disease, a subset may experience multiple progressive diseases with rapid deterioration, rendering them unsuitable for salvage local therapy and leading to poorer prognosis. In our study, preliminary survival benefits were observed in patients receiving LCT compared to those who did not, among whom salvage local therapies could have been administered. Future clinical trials are necessary to compare the safety and efficacy of LCT and salvage local therapy in metastatic NSCLC patients receiving immunotherapy.
Our study has several limitations. Firstly, due to the nature of retrospective design and a relatively small number of patients with ORD in the current study, the potential clinical value and survival benefit of LCT among patients with ORD should be interpreted with caution since considerable selection bias could be existed. Although the baseline disease characteristics between the LCT group and the non-LCT group were generally balanced, there may be some crucial baseline parameters missed. Nevertheless, relevant clinical data about the clinical value of LCT in immunotherapy-treated NSCLC is currently scarce. There has been a growing need to obtain a proper combinational strategy to overcome acquired resistance in NSCLC patients receiving PD-1/PD-L1 inhibitors. This study provided preliminary but valuable information about the rationale and potential clinical value of LCT in PD-1/PD-L1-inhibitor-treated NSCLC with ORD. Secondly, it was difficult for us to collect the comprehensive and accurate information about the safety profiles of the combinational therapy of LCT and immunotherapy. In the current study, only grade 3–5 treatment-related AEs were documented, which were generally consistent with previous studies reporting promising results about the safety profiles of the combination therapy of PD-1/PD-L1 inhibitors with radiotherapy, especially hypo fractionated radiotherapy and SBRT [41,42,43]. In a recent phase 2 trial, upfront local ablative therapy consisting of radiotherapy, surgery and radiofrequency ablation, combing with pembrolizumab, resulted in generally comparable incidence of treatment-related toxicities with those receiving pembrolizumab monotherapy in oligo-metastatic NSCLC [41]. Grade ≥ 2 treatment-related adverse events occurred in 25% of the patients receiving hypofractionated radiotherapy and immunotherapy in a pooled analysis of two prospective trials, which was generally manageable [44]. Similarly, a meta-analysis including 51 studies with 15,398 patients found comparable grade 3–4 toxicities in those receiving both immunotherapy and radiotherapy (16.3%; 95% CI, 11.1–22.3%) and those receiving immunotherapy alone (22.3%; 95% CI, 18.1–26.9%) [43].
Conclusions
Oligo-residual disease (ORD) was observed in approximately 20% of patients undergoing treatment with PD-1/PD-L1 inhibitors. Moreover, local consolidative therapy (LCT) appears to offer additional survival benefits for those exhibiting ORD following effective PD-1/PD-L1 inhibitor therapy. Nonetheless, the validation of this observation in future prospective studies with larger sample sizes is imperative.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leal TA, Riess JW, Jensen E, Zhao B, Pietanza MC, Brahmer JR (2021) Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol 39:2339–2349. https://doi.org/10.1200/jco.21.00174
Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, Hui R, Hochmair MJ, Clingan P, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Garon EB, Novello S, Rubio-Viqueira B, Boyer M, Kurata T, Gray JE, Yang J, Bas T, Pietanza MC, Garassino MC (2020) Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 38:1505–1517. https://doi.org/10.1200/jco.19.03136
Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, Hermes B, Cicin I, Medgyasszay B, Rodríguez-Cid J, Okamoto I, Lee S, Ramlau R, Vladimirov V, Cheng Y, Deng X, Zhang Y, Bas T, Piperdi B, Halmos B (2020) A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol 15:1657–1669. https://doi.org/10.1016/j.jtho.2020.06.015
Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro CJ, Pluzanski A, Arrieta O, Frontera OA, Chiari R, Butts C, Wójcik-Tomaszewska J, Coudert B, Garassino MC, Ready N, Felip E, García MA, Waterhouse D, Domine M, Barlesi F, Antonia S, Wohlleber M, Gerber DE, Czyzewicz G, Spigel DR, Crino L, Eberhardt WEE, Li A, Marimuthu S, Brahmer J (2021) Five-year outcomes from the randomized, phase III trials checkmate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol 39:723–733. https://doi.org/10.1200/jco.20.01605
Passaro A, Brahmer J, Antonia S, Mok T, Peters S (2022) Managing resistance to immune checkpoint inhibitors in lung cancer: treatment and novel strategies. J Clin Oncol 40:598–610. https://doi.org/10.1200/jco.21.01845
Schoenfeld AJ, Hellmann MD (2020) Acquired resistance to immune checkpoint inhibitors. Cancer Cell 37:443–455. https://doi.org/10.1016/j.ccell.2020.03.017
Hellman S, Weichselbaum RR (1995) Oligometastases. J Clin Oncol 13:8–10. https://doi.org/10.1200/jco.1995.13.1.8
Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, Dingemans AC, Fournier B, Hurkmans C, Lecouvet FE, Meattini I, Méndez Romero A, Ricardi U, Russell NS, Schanne DH, Scorsetti M, Tombal B, Verellen D, Verfaillie C, Ost P (2020) Characterisation and classification of oligometastatic disease: a European society for radiotherapy and oncology and European organisation for research and treatment of cancer consensus recommendation. Lancet Oncol 21:e18–e28. https://doi.org/10.1016/s1470-2045(19)30718-1
Xu Q, Zhou F, Liu H, Jiang T, Li X, Xu Y, Zhou C (2018) Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol 13:1383–1392. https://doi.org/10.1016/j.jtho.2018.05.019
Guo T, Ni J, Yang X, Li Y, Li Y, Zou L, Wang S, Liu Q, Chu L, Chu X, Li S, Ye L, Zhu Z (2020) Pattern of recurrence analysis in metastatic EGFR-mutant NSCLC treated with osimertinib: implications for consolidative stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 107:62–71. https://doi.org/10.1016/j.ijrobp.2019.12.042
Dall’Olio FG, Marabelle A, Caramella C, Garcia C, Aldea M, Chaput N, Robert C, Besse B (2022) Tumour burden and efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol 19:75–90. https://doi.org/10.1038/s41571-021-00564-3
Chandra RA, Keane FK, Voncken FEM, Thomas CR Jr (2021) Contemporary radiotherapy: present and future. Lancet 398:171–184. https://doi.org/10.1016/s0140-6736(21)00233-6
Gomez DR, Blumenschein GR Jr, Lee JJ, Hernandez M, Ye R, Camidge DR, Doebele RC, Skoulidis F, Gaspar LE, Gibbons DL, Karam JA, Kavanagh BD, Tang C, Komaki R, Louie AV, Palma DA, Tsao AS, Sepesi B, William WN, Zhang J, Shi Q, Wang XS, Swisher SG, Heymach JV (2016) Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 17:1672–1682. https://doi.org/10.1016/s1470-2045(16)30532-0
Gomez DR, Tang C, Zhang J, Blumenschein GR Jr, Hernandez M, Lee JJ, Ye R, Palma DA, Louie AV, Camidge DR, Doebele RC, Skoulidis F, Gaspar LE, Welsh JW, Gibbons DL, Karam JA, Kavanagh BD, Tsao AS, Sepesi B, Swisher SG, Heymach JV (2019) Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II. Randomized Study J Clin Oncol 37:1558–1565. https://doi.org/10.1200/jco.19.00201
Zeng Y, Ni J, Yu F, Zhou Y, Zhao Y, Li S, Guo T, Chu L, Yang X, Chu X, Cai X, Zhu Z (2020) The value of local consolidative therapy in Osimertinib-treated non-small cell lung cancer with oligo-residual disease. Radiat Oncol 15:207. https://doi.org/10.1186/s13014-020-01651-y
Chan OSH, Lam KC, Li JYC, Choi FPT, Wong CYH, Chang ATY, Mo FKF, Wang K, Yeung RMW, Mok TSK (2020) ATOM: a phase II study to assess efficacy of preemptive local ablative therapy to residual oligometastases of NSCLC after EGFR TKI. Lung Cancer 142:41–46. https://doi.org/10.1016/j.lungcan.2020.02.002
Peeters STH, Van Limbergen EJ, Hendriks LEL, De Ruysscher D (2021) Radiation for oligometastatic lung cancer in the era of immunotherapy: What do we (need to) know? Cancers (Basel). https://doi.org/10.3390/cancers13092132
Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley DA, Kranz DM, Schreiber H (2007) Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med 204:49–55. https://doi.org/10.1084/jem.20062056
Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, Demaria S (2008) Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 181:3099–3107. https://doi.org/10.4049/jimmunol.181.5.3099
Jiang S, Zhang J, Chu L, Chu X, Yang X, Li Y, Guo T, Zhou Y, Xu D, Mao J, Zheng Z, An Y, Sun H, Dong H, Yu S, Ye R, Hu J, Chu Q, Ni J, Zhu Z (2022) Atypical response in metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors: radiographic patterns and clinical value of local therapy. Cancers (Basel). https://doi.org/10.3390/cancers15010180
Dingemans AC, Hendriks LEL, Berghmans T, Levy A, Hasan B, Faivre-Finn C, Giaj-Levra M, Giaj-Levra N, Girard N, Greillier L, Lantuéjoul S, Edwards J, O’Brien M, Reck M, Smit EF, Van Schil P, Postmus PE, Ramella S, Lievens Y, Gaga M, Peled N, Scagliotti GV, Senan S, Paz-Ares L, Guckenberger M, McDonald F, Ekman S, Cufer T, Gietema H, Infante M, Dziadziuszko R, Peters S, Porta RR, Vansteenkiste J, Dooms C, de Ruysscher D, Besse B, Novello S (2019) Definition of synchronous oligometastatic non-small cell lung cancer-A consensus report. J Thorac Oncol 14:2109–2119. https://doi.org/10.1016/j.jtho.2019.07.025
Miyawaki T, Kenmotsu H, Kodama H, Nishioka N, Miyawaki E, Mamesaya N, Kobayashi H, Omori S, Ko R, Wakuda K, Ono A, Naito T, Murakami H, Mori K, Harada H, Endo M, Takahashi K, Takahashi T (2021) Association between oligo-residual disease and patterns of failure during EGFR-TKI treatment in EGFR-mutated non-small cell lung cancer: a retrospective study. BMC Cancer 21:1247. https://doi.org/10.1186/s12885-021-08983-2
Zhang Y, Chang L, Yang Y, Fang W, Guan Y, Wu A, Hong S, Zhou H, Chen G, Chen X, Zhao S, Zheng Q, Pan H, Zhang L, Long H, Yang H, Wang X, Wen Z, Wang J, Yang H, Xia X, Zhao Y, Hou X, Ma Y, Zhou T, Zhang Z, Zhan J, Huang Y, Zhao H, Zhou N, Yi X, Zhang L (2019) Intratumor heterogeneity comparison among different subtypes of non-small-cell lung cancer through multi-region tissue and matched ctDNA sequencing. Mol Cancer 18:7. https://doi.org/10.1186/s12943-019-0939-9
McGranahan N, Rosenthal R, Hiley CT, Rowan AJ, Watkins TBK, Wilson GA, Birkbak NJ, Veeriah S, Van Loo P, Herrero J, Swanton C (2017) Allele-specific HLA loss and immune escape in lung cancer evolution. Cell 171:1259-1271.e1211. https://doi.org/10.1016/j.cell.2017.10.001
Salcher S, Sturm G, Horvath L, Untergasser G, Kuempers C, Fotakis G, Panizzolo E, Martowicz A, Trebo M, Pall G, Gamerith G, Sykora M, Augustin F, Schmitz K, Finotello F, Rieder D, Perner S, Sopper S, Wolf D, Pircher A, Trajanoski Z (2022) High-resolution single-cell atlas reveals diversity and plasticity of tissue-resident neutrophils in non-small cell lung cancer. Cancer Cell 40:1503-1520.e1508. https://doi.org/10.1016/j.ccell.2022.10.008
Jia Q, Wu W, Wang Y, Alexander PB, Sun C, Gong Z, Cheng JN, Sun H, Guan Y, Xia X, Yang L, Yi X, Wan YY, Wang H, He J, Futreal PA, Li QJ, Zhu B (2018) Local mutational diversity drives intratumoral immune heterogeneity in non-small cell lung cancer. Nat Commun 9:5361. https://doi.org/10.1038/s41467-018-07767-w
Schoenfeld AJ, Rizvi HA, Memon D, Shaverdian N, Bott MJ, Sauter JL, Tsai CJ, Lihm J, Hoyos D, Plodkowski AJ, Perez-Johnston R, Sawan P, Egger JV, Greenbaum BD, Rimner A, Riely GJ, Rudin CM, Rusch VW, Gomez DR, Hellmann MD (2022) Systemic and oligo-acquired resistance to PD-(L)1 blockade in lung cancer. Clin Cancer Res 28:3797–3803. https://doi.org/10.1158/1078-0432.Ccr-22-0657
Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, Dowell JE, Cheedella N, Nedzi L, Westover KD, Pulipparacharuvil S, Choy H, Timmerman RD (2018) Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol 4:e173501. https://doi.org/10.1001/jamaoncol.2017.3501
Kim C, Hoang CD, Kesarwala AH, Schrump DS, Guha U, Rajan A (2017) Role of local ablative therapy in patients with oligometastatic and oligoprogressive non-small cell lung cancer. J Thorac Oncol 12:179–193. https://doi.org/10.1016/j.jtho.2016.10.012
Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC (2018) Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 18:313–322. https://doi.org/10.1038/nrc.2018.6
Daly ME, Monjazeb AM, Kelly K (2015) Clinical trials integrating immunotherapy and radiation for non-small-cell lung cancer. J Thorac Oncol 10:1685–1693. https://doi.org/10.1097/jto.0000000000000686
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX (2014) Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 124:687–695. https://doi.org/10.1172/jci67313
Ko EC, Raben D, Formenti SC (2018) The integration of radiotherapy with immunotherapy for the treatment of non-small cell lung cancer. Clin Cancer Res 24:5792–5806. https://doi.org/10.1158/1078-0432.Ccr-17-3620
Zhou Y, Yu F, Zhao Y, Zeng Y, Yang X, Chu L, Chu X, Li Y, Zou L, Guo T, Zhu Z, Ni J (2020) A narrative review of evolving roles of radiotherapy in advanced non-small cell lung cancer: from palliative care to active player. Transl Lung Cancer Res 9:2479–2493. https://doi.org/10.21037/tlcr-20-1145
Wang P, Yin T, Zhao K, Yu J, Teng F (2022) Efficacy of single-site radiotherapy plus PD-1 inhibitors vs PD-1 inhibitors for oligometastatic non-small cell lung cancer. J Cancer Res Clin Oncol 148:1253–1261. https://doi.org/10.1007/s00432-021-03849-3
Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, Adamow M, Kuk D, Panageas KS, Carrera C, Wong P, Quagliarello F, Wubbenhorst B, D’Andrea K, Pauken KE, Herati RS, Staupe RP, Schenkel JM, McGettigan S, Kothari S, George SM, Vonderheide RH, Amaravadi RK, Karakousis GC, Schuchter LM, Xu X, Nathanson KL, Wolchok JD, Gangadhar TC, Wherry EJ (2017) T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545:60–65. https://doi.org/10.1038/nature22079
Harada D, Takigawa N (2021) Oligoprogression in non-small cell lung cancer. Cancers (Basel). https://doi.org/10.3390/cancers13225823
Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA Jr, Aisner DL, Gaspar LE, Kavanagh BD, Doebele RC, Camidge DR (2012) Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 7:1807–1814. https://doi.org/10.1097/JTO.0b013e3182745948
Chan OSH, Lee VHF, Mok TSK, Mo F, Chang ATY, Yeung RMW (2017) The role of radiotherapy in epidermal growth factor receptor mutation-positive patients with oligoprogression: a matched-cohort analysis. Clin Oncol (R Coll Radiol) 29:568–575. https://doi.org/10.1016/j.clon.2017.04.035
Friedes C, Mai N, Fu W, Hu C, Hazell SZ, Han P, McNutt TR, Forde PM, Redmond KJ, Voong KR, Hales RK (2020) Isolated progression of metastatic lung cancer: clinical outcomes associated with definitive radiotherapy. Cancer 126:4572–4583. https://doi.org/10.1002/cncr.33109
Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, Deshpande C, Miller L, Patel P, Alley E, Knepley C, Mutale F, Cohen RB, Langer CJ (2019) Pembrolizumab after completion of locally ablative therapy for oligometastatic non-small cell lung cancer: A phase 2 trial. JAMA Oncol 5:1283–1290. https://doi.org/10.1001/jamaoncol.2019.1449
Anscher MS, Arora S, Weinstock C, Amatya A, Bandaru P, Tang C, Girvin AT, Fiero MH, Tang S, Lubitz R, Amiri-Kordestani L, Theoret MR, Pazdur R, Beaver JA (2022) Association of radiation therapy with risk of adverse events in patients receiving immunotherapy: a pooled analysis of trials in the US food and drug administration database. JAMA Oncol 8:232–240. https://doi.org/10.1001/jamaoncol.2021.6439
Sha CM, Lehrer EJ, Hwang C, Trifiletti DM, Mackley HB, Drabick JJ, Zaorsky NG (2020) Toxicity in combination immune checkpoint inhibitor and radiation therapy: a systematic review and meta-analysis. Radiother Oncol 151:141–148. https://doi.org/10.1016/j.radonc.2020.07.035
Popp I, Vaes RDW, Wieten L, Adebahr S, Hendriks L, Bavafaye Haghighi E, Degens J, Schäfer H, Greil C, Peeters S, Waller CF, Houben R, Niedermann G, Rawluk J, Gkika E, Duyster J, Grosu AL, De Ruysscher D (2023) Radiotherapy to reinvigorate immunotherapy activity after acquired resistance in metastatic non-small-cell lung cancer: a pooled analysis of two institutions prospective phase II single arm trials. Radiother Oncol 190:110048. https://doi.org/10.1016/j.radonc.2023.110048
Acknowledgements
We acknowledge all the patients and their families who participated in the study, the medical and nursing staff for their support, and all the members of our group for their efforts. We also acknowledge the Essaystar Group for language editing.
Funding
This work was supported by the Chinese Society of Clinical Oncology (Grant No. Y-BMS2019082, Y-MSD20200147) and the Shanghai Science and Technology Committee (Grant No. 20Y11913500).
Author information
Authors and Affiliations
Contributions
Jinmeng Zhang.Jie Gao and Jiuang Mao wrote the main manuscript text, Li Chu.Xiao Chu and Xi Yang made contributions to the conception and design of the work; Yida Li.Tiantian Guo.Yue Zhou and Dayu Xu analysis the acquisition;and Jie Hu.Qian Chu.Jianjiao Ni and Zhengfei Zhu prepared figures and Tables. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Gao, J., Jiang, S. et al. Oligo-residual disease in PD-1/PD-L1 inhibitor-treated metastatic non-small cell lung cancer: incidence, pattern of failure, and clinical value of local consolidative therapy. Cancer Immunol Immunother 73, 140 (2024). https://doi.org/10.1007/s00262-024-03720-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00262-024-03720-7