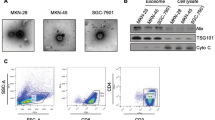
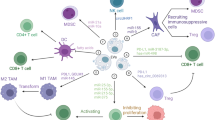
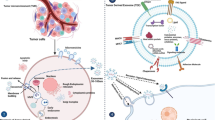
Abstract
Background
Colorectal cancer is a disease of unmet medical need. Although extracellular vesicles (EVs) have been implicated in anti-tumor responses, discrepancies were observed among studies. We analyzed the role of tumor-derived EVs (TEVs) in tumor progression in vivo by focusing on regulatory T (Treg) cells, which play essential roles in tumor development and progression.
Methods
A mouse model of colorectal cancer lung metastasis was generated using BALB/c mice by tail vein injection of the BALB/c colon adenocarcinoma cell line Colon-26. TEVs derived from Colon-26 and BALB/c lung squamous cell carcinoma ASB-XIV were retrieved from the culture media supernatants. A TEV equivalent to 10 µg protein was injected every other day for 2 weeks.
Results
Histology and immunohistochemistry studies revealed that lung tumors reduced in the Colon-26-EV group when compared to the phosphate-buffered saline (PBS) group. The population of CD4 + FoxP3 + cells in the lung was upregulated in the PBS group mice when compared to the healthy mice (P < 0.001), but was significantly downregulated in the Colon-26-EV group mice when compared to the PBS group mice (P < 0.01). Programmed cell death protein 1, glucocorticoid-induced TNFR-related protein, and CD69 expression in lung Treg cells were markedly upregulated in the PBS group when compared to the healthy mice, but downregulated in the Colon-26-EV group when compared to the PBS group. The changes in expression were dose-dependent for Colon-26-EVs. ASB-EVs also led to significantly downregulated Treg cell expression, although non-cancer line 3T3-derived EVs did not.
Conclusion
Our study suggests that TEVs possess components for tumor suppression.






Similar content being viewed by others
Data availability
The datasets of this study are available from the corresponding author upon reasonable request.
Abbreviations
- CFSE:
-
Carboxyfluorescein succinimidyl ester
- CRC:
-
Colorectal cancer
- DC:
-
Dendritic cells
- EV:
-
Extracellular vesicle
- FBS:
-
Fetal bovine serum
- H&E:
-
Hematoxylin and eosin
- ICI:
-
Immune checkpoint inhibitor
- NTA:
-
Nanoparticle tracking analysis
- PBS:
-
Phosphate-buffered saline
- Treg:
-
Regulatory T
- TEM:
-
Transmission electron microscopy
- TME:
-
Tumor microenvironment
- TEVs:
-
Tumor-derived EVs
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA (2010) Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 116:544–573. https://doi.org/10.1002/cncr.24760
Xie YH, Chen YX, Fang JY (2020) Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther 5:22. https://doi.org/10.1038/s41392-020-0116-z
Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA (2019) Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 16:361–375. https://doi.org/10.1038/s41575-019-0126-x
Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 133:775–787. https://doi.org/10.1016/j.cell.2008.05.009
Shang B, Liu Y, Jiang SJ, Liu Y (2015) Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep 5:15179. https://doi.org/10.1038/srep15179
Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O (2015) Biological properties of extracellular vesicles and their physiological functions Yáñez-Mó M. J Extracell Vesicles 4:27066
Roefs MT, Sluijter JPG, Vader P (2020) Extracellular vesicle-associated proteins in tissue repair. Trends Cell Biol 30:990–1013. https://doi.org/10.1016/j.tcb.2020.09.009
Zhou X, Xie F, Wang L, Zhang L, Zhang S, Fang M, Zhou F (2020) The function and clinical application of extracellular vesicles in innate immune regulation. Cell Mol Immunol 17:323–334. https://doi.org/10.1038/s41423-020-0391-1
Ko SY, Lee W, Kenny HA, Dang LH, Ellis LM, Jonasch E, Lengyel E, Naora H (2019) Cancer-derived small extracellular vesicles promote angiogenesis by heparin-bound, bevacizumab-insensitive VEGF, independent of vesicle uptake. Commun Biol 2:386. https://doi.org/10.1038/s42003-019-0609-x
Cui Y, Wang D, Xie M (2021) Tumor-derived extracellular vesicles promote activation of carcinoma-associated fibroblasts and facilitate invasion and metastasis of ovarian cancer by carrying miR-630. Front Cell Dev Biol 9:652322. https://doi.org/10.3389/fcell.2021.652322
Jung T, Castellana D, Klingbeil P, Cuesta Hernández I, Vitacolonna M, Orlicky DJ, Roffler SR, Brodt P, Zöller M (2009) CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia 11:1093–1105. https://doi.org/10.1593/neo.09822
Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527:329–335. https://doi.org/10.1038/nature15756
Bardi GT, Smith MA, Smith HJLGT, Bardi (2018) Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine 105:63–72. https://doi.org/10.1016/j.cyto.2018.02.002
Liu J, Wu S, Zheng X, Zheng P, Fu Y, Wu C, Lu B, Ju J, Jiang J (2020) Immune suppressed tumor microenvironment by exosomes derived from gastric cancer cells via modulating immune functions. Sci Rep 10:14749. https://doi.org/10.1038/s41598-020-71573-y
Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L (2001) Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7:297–303. https://doi.org/10.1038/85438
Altieri SL, Khan AN, Tomasi TB (2004) Exosomes from plasmacytoma cells as a tumor vaccine. J Immunother 27:282–288. https://doi.org/10.1097/00002371-200407000-00004
Wang DY, Ye F, Lin JJ, Xu X (2017) Cutaneous metastasis: a rare phenomenon of colorectal cancer. Ann Surg Treat Res 93:277–280. https://doi.org/10.4174/astr.2017.93.5.277
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan M, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ, Kornek M, Kosanović MM, Kovács Á, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz Á, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7:1535750. https://doi.org/10.1080/20013078.2018.1535750
Rayamajhi M, Redente EF, Condon TV, Gonzalez-Juarrero M, Riches DW, Lenz LL (2011) Non-surgical intratracheal instillation of mice with analysis of lungs and lung draining lymph nodes by flow cytometry. J Vis Exp 51. https://doi.org/10.3791/2702
Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B (2012) TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLOS ONE 7:e30676. https://doi.org/10.1371/journal.pone.0030676
Katano T, Fukuda M, Furue H, Yamazaki M, Abe M, Watanabe M, Nishida K, Yao I, Yamada A, Hata Y, Okumura N, Nakazawa T, Yamamoto T, Sakimura K, Takao T, Ito S (2016) Involvement of brain-enriched guanylate kinase-associated protein (BEGAIN) in chronic pain after peripheral nerve injury. eNeuro 3. https://doi.org/10.1523/ENEURO.0110-16.2016
Saleh R, Elkord E (2020) FoxP3+ T regulatory cells in cancer: prognostic biomarkers and therapeutic targets. Cancer Lett 490:174–185. https://doi.org/10.1016/j.canlet.2020.07.022
Glémain A, Néel M, Néel A, André-Grégoire G, Gavard J, Martinet B, Le Bloas R, Riquin K, Hamidou M, Fakhouri F, Bruneau S (2022) Neutrophil-derived extracellular vesicles induce endothelial inflammation and damage through the transfer of miRNAs. J Autoimmun 129:102826. https://doi.org/10.1016/j.jaut.2022.102826
Zhou H, Shen X, Yan C, Xiong W, Ma Z, Tan Z, Wang J, Li Y, Liu J, Duan A, Liu F (2022) Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate osteoarthritis of the knee in mice model by interacting with METTL3 to reduce m6A of NLRP3 in macrophage. Stem Cell Res Ther 13:322. https://doi.org/10.1186/s13287-022-03005-9
Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T (2014) Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 7:ra63. https://doi.org/10.1126/scisignal.2005231
Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H (2013) Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol 49:503–510. https://doi.org/10.1165/rcmb.2013-0086MA
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31:164–172. https://doi.org/10.1016/j.tibs.2006.01.006
Rosenzweig R, Nillegoda NB, Mayer MP, Bukau B (2019) The Hsp70 chaperone network. Nat Rev Mol Cell Biol 20:665–680. https://doi.org/10.1038/s41580-019-0133-3
Kasioumi P, Vrazeli P, Vezyraki P, Zerikiotis S, Katsouras C, Damalas A, Angelidis C (2019) Hsp70 (HSP70A1A) downregulation enhances the metastatic ability of cancer cells. Int J Oncol 54:821–832. https://doi.org/10.3892/ijo.2018.4666
Tsan MF, Gao B (2004) Heat shock protein and innate immunity. Cell Mol Immunol 1:274–279
Multhoff G, Mizzen L, Winchester CC, Milner CM, Wenk S, Eissner G, Kampinga HH, Laumbacher B, Johnson J (1999) Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol 27:1627–1636. https://doi.org/10.1016/s0301-472x(99)00104-6
Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK (1997) Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med 186:1315–1322. https://doi.org/10.1084/jem.186.8.1315
Ueda G, Tamura Y, Hirai I, Kamiguchi K, Ichimiya S, Torigoe T, Hiratsuka H, Sunakawa H, Sato N (2004) Tumor-derived heat shock protein 70-pulsed dendritic cells elicit tumor-specific cytotoxic T lymphocytes (CTLs) and tumor immunity. Cancer Sci 95:248–253. https://doi.org/10.1111/j.1349-7006.2004.tb02211.x
Komarova EY, Suezov RV, Nikotina AD, Aksenov ND, Garaeva LA, Shtam TA, Zhakhov AV, Martynova MG, Bystrova OA, Istomina MS, Ischenko AM, Margulis BA, Guzhova IV (2021) Hsp70-containing extracellular vesicles are capable of activating of adaptive immunity in models of mouse melanoma and colon carcinoma. Sci Rep 11:21314. https://doi.org/10.1038/s41598-021-00734-4
Cho JA, Lee YS, Kim SH, Ko JK, Kim CW (2009) MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer Lett 275:256–265. https://doi.org/10.1016/j.canlet.2008.10.021
Piersiala K, Neves F, da Silva P, Hjalmarsson E, Kolev A, Kågedal Å, Starkhammar M, Elliot A, Marklund L, Margolin G, Munck-Wikland E, Kumlien Georén S, Cardell LO (2021) CD4+ and CD8+ T cells in sentinel nodes exhibit distinct pattern of PD-1, CD69, and HLA-DR expression compared to tumor tissue in oral squamous cell carcinoma. Cancer Sci 112:1048–1059. https://doi.org/10.1111/cas.14816
Kim HR, Park HJ, Son J, Lee JG, Chung KY, Cho NH, Shim HS, Park S, Kim G, In Yoon H, Kim HG, Jung YW, Cho BC, Park SY, Rha SY, Ha SJ (2019) Tumor microenvironment dictates regulatory T cell phenotype: upregulated immune checkpoints reinforce suppressive function. J Immunother Cancer 7:339. https://doi.org/10.1186/s40425-019-0785-8
Ma F, Vayalil J, Lee G, Wang Y, Peng G (2021) Emerging role of tumor-derived extracellular vesicles in T cell suppression and dysfunction in the tumor microenvironment. J Immunother Cancer 9. https://doi.org/10.1136/jitc-2021-003217
Nakazawa Y, Nishiyama N, Koizumi H, Kanemaru K, Nakahashi-Oda C, Shibuya A (2021) Tumor-derived extracellular vesicles regulate tumor-infiltrating regulatory T cells via the inhibitory immunoreceptor CD300a. eLife 10. https://doi.org/10.7554/eLife.61999
Yamada N, Kuranaga Y, Kumazaki M, Shinohara H, Taniguchi K, Akao Y (2016) Colorectal cancer cell-derived extracellular vesicles induce phenotypic alteration of T cells into tumor-growth supporting cells with transforming growth factor-β1-mediated suppression. Oncotarget 7:27033–27043. https://doi.org/10.18632/oncotarget.7041
Wachstein J, Tischer S, Figueiredo C, Limbourg A, Falk C, Immenschuh S, Blasczyk R, Eiz-Vesper B (2012) HSP70 enhances immunosuppressive function of CD4(+)CD25(+)FoxP3(+) T regulatory cells and cytotoxicity in CD4(+)CD25(-) T cells. PLOS ONE 7:e51747. https://doi.org/10.1371/journal.pone.0051747
Fang H, Wu Y, Huang X, Wang W, Ang B, Cao X, Wan T (2011) Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J Biol Chem 286:30393–30400. https://doi.org/10.1074/jbc.M111.266528
Wang C, Huang X, Wu Y, Wang J, Li F, Guo G (2020) Tumor cell-associated exosomes robustly elicit anti-tumor immune responses through modulating dendritic cell vaccines in lung tumor. Int J Biol Sci 16:633–643. https://doi.org/10.7150/ijbs.38414
Kugeratski FG, McAndrews KM, Kalluri R (2021) Multifunctional applications of engineered extracellular vesicles in the treatment of cancer. Endocrinology 162. https://doi.org/10.1210/endocr/bqaa250
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This study was funded by (1) Grant-in-Aid for Young Scientists JSPS KAKENHI Grant Number JP20K17066, (2) Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, (3) Grant-in-Aid for Scientific Research (C) of the Ministry of Culture and Science of Japan (20590810, 23591017, 24591020, 12008507, 17877850, 17K09468, 15K09052), (4) the Research Program on Intractable Diseases, from the Ministry of Labor and Welfare of Japan, and (5) grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, (6) the Research Program from the Japan Medical Research and Development (AMED) (17824893).
Author information
Authors and Affiliations
Contributions
SK and TT designed the study and wrote the initial draft of the manuscript. SK, TT, NK, NN, MM, and YM performed the experiments. YH, TT, TI, TF, KO, and MN contributed to the analysis and interpretation of data and assisted in the preparation of the manuscript. All other authors have contributed to data collection and interpretation and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no competing interests to declare.
Research involving Animals
This research has been approved by Animal Ethics Committee (#18-115(01)) and Recombinant DNA Experiments Committee (#30-012-1) of Kansai Medical University.
Informed consent
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kobayashi, S., Kondo, N., Tomiyama, T. et al. Intravenous injection of tumor extracellular vesicles suppresses tumor growth by reducing the regulatory T cell phenotype. Cancer Immunol Immunother 72, 3651–3664 (2023). https://doi.org/10.1007/s00262-023-03517-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03517-0