Abstract
Background
This study aimed to assess the efficacy and safety of geptanolimab (GB226), a fully humanized, recombinant anti-programmed cell death-1 monoclonal antibody, in Chinese patients with refractory or relapsed (r/r) primary mediastinal large B-cell lymphoma (PMBCL).
Methods
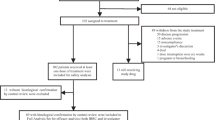
This was a multicenter, open-label, single-arm phase II study (Gxplore-003), conducted at 43 hospitals in China (NCT03639181). Patients received geptanolimab intravenously at a dose of 3 mg/kg every 2 weeks until documented confirmed disease progression, intolerable toxicity, or any other cessation criteria was met. The primary endpoint was objective response rate (ORR) in the full analysis set assessed by the independent review committee (IRC) according to the Lugano Classification 2014.
Results
This study was prematurely terminated due to the slow rate of patient accrual. Between Oct 15th, 2018 and Oct 7th, 2020, 25 patients were enrolled and treated. By the data cutoff date on Dec 23rd, 2020, the IRC-assessed ORR was 68.0% (17/25; 95% confidence interval [CI] 46.5–85.1%), with the complete response rate of 24%. The disease control rate was 88% (22/25; 95%CI 68.8–97.5%). Median duration of response was not reached (NR) (95%CI, 5.62 months to NR), with 79.5% of patients having response durations of more than 12 months. Median progression-free survival was NR (95%CI, 6.83 months to NR). Treatment-related adverse events (TRAEs) were reported in 20 of 25 (80.0%) patients, and grade 3 or higher TRAEs occurred in 11 of 25 (44%) patients. No treatment-related deaths occurred. The immune-related adverse events (irAEs) of any grade were observed in 6 (24.0%) patients, and no grade 4 or grade 5 irAEs were reported.
Conclusion
Geptanolimab (GB226) demonstrated promising efficacy and a manageable safety profile in Chinese patients with r/r PMBCL.




Similar content being viewed by others
Data availability
Data generated and analyzed in this study are on file with Genor Biopharma Co., Ltd., Shanghai, China and are not publicly available according to the company.
References
Alaggio R, Amador C, Anagnostopoulos I et al. (2022) The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia, 36:1720–1748. Doi: https://doi.org/10.1038/s41375-022-01620-2
Rosenwald A, Wright G, Leroy K et al (2003) Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 198:851–862. https://doi.org/10.1084/jem.20031074
Savage KJ, Monti S, Kutok JL et al (2003) The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood 102:3871–3879. https://doi.org/10.1182/blood-2003-06-1841
Giulino-Roth L, O’Donohue T, Chen Z et al (2017) Outcomes of adults and children with primary mediastinal B-cell lymphoma treated with dose-adjusted EPOCH-R. Br J Haematol 179:739–747. https://doi.org/10.1111/bjh.14951
Hayden AR, Tonseth P, Lee DG et al (2020) Outcome of primary mediastinal large B-cell lymphoma using R-CHOP: impact of a PET-adapted approach. Blood 136:2803–2811. https://doi.org/10.1182/blood.2019004296
Martelli M, Ceriani L, Zucca E et al (2014) [18F]fluorodeoxyglucose positron emission tomography predicts survival after chemoimmunotherapy for primary mediastinal large B-cell lymphoma: results of the International Extranodal Lymphoma Study Group IELSG-26 Study. J Clin Oncol 32:1769–1775. https://doi.org/10.1200/JCO.2013.51.7524
Melani C, Advani R, Roschewski M et al (2018) End-of-treatment and serial PET imaging in primary mediastinal B-cell lymphoma following dose-adjusted EPOCH-R: a paradigm shift in clinical decision making. Haematologica 103:1337–1344. https://doi.org/10.3324/haematol.2018.192492
Rieger M, Osterborg A, Pettengell R et al (2011) Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol 22:664–670. https://doi.org/10.1093/annonc/mdq418
Savage KJ, Al-Rajhi N, Voss N, Paltiel C, Klasa R, Gascoyne RD, Connors JM (2006) Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol 17:123–130. https://doi.org/10.1093/annonc/mdj030
Kuruvilla J, Pintilie M, Tsang R, Nagy T, Keating A, Crump M (2008) Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk Lymphoma 49:1329–1336. https://doi.org/10.1080/10428190802108870
Aoki T, Shimada K, Suzuki R et al (2015) High-dose chemotherapy followed by autologous stem cell transplantation for relapsed/refractory primary mediastinal large B-cell lymphoma. Blood Cancer J 5:e372. https://doi.org/10.1038/bcj.2015.101
Zhou H, Xu-Monette ZY, Xiao L et al (2020) Prognostic factors, therapeutic approaches, and distinct immunobiologic features in patients with primary mediastinal large B-cell lymphoma on long-term follow-up. Blood Cancer J 10:49. https://doi.org/10.1038/s41408-020-0312-7
Steidl C, Gascoyne RD (2011) The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood 118:2659–2669. https://doi.org/10.1182/blood-2011-05-326538
Zinzani PL, Pellegrini C, Chiappella A, Di Rocco A, Salvi F, Cabras MG, Argnani L, Stefoni V (2017) Brentuximab vedotin in relapsed primary mediastinal large B-cell lymphoma: results from a phase 2 clinical trial. Blood 129:2328–2330. https://doi.org/10.1182/blood-2017-01-764258
Neelapu SS, Locke FL, Bartlett NL et al (2017) Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377:2531–2544. https://doi.org/10.1056/NEJMoa1707447
Locke FL, Ghobadi A, Jacobson CA et al (2019) Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 20:31–42. https://doi.org/10.1016/S1470-2045(18)30864-7
Abramson JS, Palomba ML, Gordon LI et al (2020) Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396:839–852. https://doi.org/10.1016/S0140-6736(20)31366-0
Savage KJ (2022) Primary mediastinal large B-cell lymphoma. Blood 140:955–970. https://doi.org/10.1182/blood.2020008376
Green MR, Monti S, Rodig SJ et al (2010) Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 116:3268–3277. https://doi.org/10.1182/blood-2010-05-282780
Twa DD, Chan FC, Ben-Neriah S et al (2014) Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood 123:2062–2065. https://doi.org/10.1182/blood-2013-10-535443
Armand P, Rodig S, Melnichenko V et al (2019) Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma. J Clin Oncol 37:3291–3299. https://doi.org/10.1200/JCO.19.01389
Zinzani PL, Ribrag V, Moskowitz CH et al (2017) Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood 130:267–270. https://doi.org/10.1182/blood-2016-12-758383
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3068. https://doi.org/10.1200/JCO.2013.54.8800
Brahmer JR, Lacchetti C, Thompson JA (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. J oncol Pract 14:247–249. https://doi.org/10.1200/jop.18.00005
Shi Y, Chen H, Qin Y et al (2023) Clinical characteristics and treatment outcomes of Chinese diffuse large B-cell lymphoma patients in the era of rituximab (2005–2018)☆. Cancer Pathog Ther 1:3–11
Chen H, Zhou Y, Han X, Shi Y (2021) The changing landscape of anti-lymphoma drug clinical trials in mainland China in the past 15 years (2005–2020): a systematic review. Lancet Reg Health West Pac 8:100097. https://doi.org/10.1016/j.lanwpc.2021.100097
Shi Y (2018) Current status and progress of lymphoma management in China. Int J Hematol 107:405–412. https://doi.org/10.1007/s12185-018-2404-8
Kline J, Godfrey J, Ansell SM (2020) The immune landscape and response to immune checkpoint blockade therapy in lymphoma. Blood 135:523–533. https://doi.org/10.1182/blood.2019000847
Mottok A, Woolcock B, Chan FC et al (2015) Genomic alterations in CIITA are frequent in primary mediastinal large B cell lymphoma and are associated with diminished MHC class II expression. Cell Rep 13:1418–1431. https://doi.org/10.1016/j.celrep.2015.10.008
Steidl C, Shah SP, Woolcock BW et al (2011) MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature 471:377–381. https://doi.org/10.1038/nature09754
Chapuy B, Stewart C, Dunford AJ et al (2019) Genomic analyses of PMBL reveal new drivers and mechanisms of sensitivity to PD-1 blockade. Blood 134:2369–2382. https://doi.org/10.1182/blood.2019002067
Le DT, Uram JN, Wang H et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509–2520. https://doi.org/10.1056/NEJMoa1500596
Wang S, Jia M, He Z, Liu XS (2018) APOBEC3B and APOBEC mutational signature as potential predictive markers for immunotherapy response in non-small cell lung cancer. Oncogene 37:3924–3936. https://doi.org/10.1038/s41388-018-0245-9
Shi Y, Su H, Song Y et al (2019) Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol 6:e12–e19. https://doi.org/10.1016/S2352-3026(18)30192-3
Su H, Song Y, Jiang W et al (2020) Sintilimab for relapsed/refractory classical Hodgkin’s lymphoma: long-term follow-up on the multicenter, single-arm phase II ORIENT-1 study. J Clin Oncol 38:8034. https://doi.org/10.1200/JCO.2020.38.15_suppl.8034
Shi Y, Cai Q, Jiang Y et al (2020) Activity and safety of geptanolimab (GB226) for patients with unresectable, recurrent, or metastatic alveolar soft part sarcoma: a phase II. Single-arm Study Clin Cancer Res 26:6445–6452. https://doi.org/10.1158/1078-0432.CCR-20-2819
Shi Y, Wu J, Wang Z et al (2021) Efficacy and safety of geptanolimab (GB226) for relapsed or refractory peripheral T cell lymphoma: an open-label phase 2 study (Gxplore-002). J Hematol Oncol 14:12. https://doi.org/10.1186/s13045-021-01033-1
Zinzani PL, Santoro A, Gritti G et al (2019) Nivolumab combined with brentuximab vedotin for relapsed/refractory primary mediastinal large B-Cell lymphoma: efficacy and safety from the phase II CheckMate 436 study. J Clin Oncol 37:3081–3089. https://doi.org/10.1200/JCO.19.01492
Chen R, Zinzani PL, Fanale MA et al (2017) Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 35:2125–2132. https://doi.org/10.1200/JCO.2016.72.1316
Younes A, Santoro A, Shipp M et al (2016) Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 17:1283–1294. https://doi.org/10.1016/S1470-2045(16)30167-X
Acknowledgements
We thank for all the patients and their families, all the investigators and study teams that contributed to this study. The authors would also like to thank Dr. Haizhu Chen (National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, China) for providing medical writing assistance with this article, funded by Genor Biopharma Co., Ltd., Shanghai, China.
Funding
This study was funded by Genor Biopharma Co., Ltd., Shanghai, China and partly supported by China National Major Project for New Drug Innovation (2017ZX09304015).
Author information
Authors and Affiliations
Contributions
YKS was the leading principal investigator, and contributed to the conceptualization and design of this study, study supervision, data acquisition and interpretation, manuscript writing and revision. YKS, JC, HZ, XHZ, LQZ, JNC, YHG, CJ, XLL, HL, ZGP, LPX, HLZ, WHZ, HYZ, LYZ and FZ contributed to patient recruitment and data acquisition. GG and WDH contributed to data collection, data analysis and data interpretation. All authors had full access to the data in this study, reviewed and approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of interest
Genny Guo and Wenduo He are employees of Genor Biopharma Co., Ltd., Shanghai, China. All other authors declare no competing interests.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation guidelines for Good Clinical Practice. The study protocol, amendments, and patient informed consent were approved by the independent ethics committee at each participating hospital.
Consent to participate
Written informed consent was obtained from all patients before screening.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Y., Cui, J., Zhou, H. et al. Efficacy and safety of geptanolimab (GB226) for relapsed/refractory primary mediastinal large B-cell lymphoma: an open-label phase II study (Gxplore-003). Cancer Immunol Immunother 72, 2991–3002 (2023). https://doi.org/10.1007/s00262-023-03467-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03467-7