Abstract
Background
A programmed cell death protein-1 (PD-1) inhibitor combined with lenvatinib and Gemox chemotherapy as first-line therapy demonstrated high anti-tumor activity against biliary tract cancer in phase II clinical trials. Herein, we aimed to investigate the efficacy and safety for advanced intrahepatic cholangiocarcinoma (ICC) in a multicenter real-world study.
Methods
Patients with advanced ICC who received PD-1 inhibitor combined with lenvatinib and Gemox chemotherapy were retrospectively screened at two medical centers. The primary endpoints were overall survival (OS) and progression-free survival (PFS), whereas the secondary endpoints were objective response rate (ORR), disease control rate (DCR), and safety. Prognostic factors for survival were analyzed.
Results
Fifty-three patients with advanced ICC were included in this study. The median follow-up time was 13.7 (95% confidence interval (CI): 12.9–17.2) months. The median OS and PFS were 14.3 (95% CI: 11.3–NR) and 8.63 (95% CI: 7.17–11.6) months, respectively. The ORR, DCR, and clinical benefit rate were 52.8, 94.3, and 75.5%, respectively. In the multivariate analysis, the tumor burden score (TBS), tumor-node metastasis classification (TNM) stage, and PD-L1 expression were independent prognostic factors for OS and PFS. All patients experienced adverse events (AEs), 41.5% (22/53) experienced grade 3 or 4 AEs, including fatigue (8/53, 15.1%) and myelosuppression (7/53, 13.2%). No grade 5 AEs were reported.
Conclusion
PD-1 inhibitors combined with lenvatinib and Gemox chemotherapy represent an effective and tolerable regimen for advanced ICC in a multicenter retrospective real-world study. TBS, TNM stage, and PD-L1 expression can be used as potential prognostic factors for OS and PFS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is one of the most common biliary tract cancers (BTC), with hidden onset, high malignancy, strong invasion, and poor prognosis [1, 2]. Surgical resection is the best treatment option for patients with early ICC. However, early ICC diagnosis lacks sufficient specificity and sensitivity. Systemic therapy is the primary treatment option for advanced ICC. Gemcitabine, platinum, fluorouracil, and albumin-bound paclitaxel are the main chemotherapeutic agents used as the first-line chemotherapy for advanced BTC. The ABC-002 study established gemcitabine plus cisplatin (GC) as the standard first-line treatment, with a median overall survival (OS) and progression-free survival (PFS) of 11.7 and 8.0 months in the GC group, respectively [3]. The ABC-06 study confirmed that folinic acid, fluorouracil, and oxaliplatin (FOLFOX) chemotherapy could be used as a second-line regimen for BTC, and the median OS of patients in the FOLFOX chemotherapy group was prolonged (6.2 vs. 5.3 months) compared with the active symptom control group [4]. Generally, the overall effect of chemotherapy is limited, and once patients develop resistance or disease progression, the treatment options are limited.
With continuous research on immune checkpoint inhibitors (ICIs), targeted therapy, and related combination therapy, more options have been provided for the treatment of advanced BTC, including ICC [5,6,7,8,9,10]. Drug resistance is one of the reasons that limit the efficacy of antitumor treatments. Combining drugs with different mechanisms of action may help overcome multiple drug resistance mechanisms. Most chemotherapeutic agents act through their direct cytotoxic effects without considering their impact on the immune system, and chemotherapy-resistant patients respond to chemotherapy rechallenge after anti-PD-1 therapy [11]. Chemotherapy can increase the response to immunotherapy by increasing the immunogenicity of tumor cells or inhibiting the immunosuppressive circuit [12, 13]. Lenvatinib is a multi-targeted tyrosine kinase inhibitor that targets vascular endothelial growth factor receptors (VEGFR1-3) and participates in the immune response by playing a role in the VEGF-VEGFR pathway [14, 15], suggesting that combination treatment with different mechanisms may play a promising role in advanced ICC.
A phase II study of tislelizumab, a PD-1 inhibitor, combined with lenvatinib, oxaliplatin, and gemcitabine (Gemox) chemotherapy used as first-line treatment for potentially resectable locally advanced BTC showed an objective response rate (ORR) of 56% and a conversion surgical resection rate of 52% [16]. Another phase II clinical trial suggested that lenvatinib combined with toripalimab, a PD-1 inhibitor, plus Gemox chemotherapy as first-line therapy, showed good efficacy in advanced ICC, with an ORR of 80% and a median PFS of 10.0 months [17]. These studies suggest that triple therapy (PD-1 inhibitors combined with lenvatinib and Gemox chemotherapy) have good efficacy for BTC. Recently, our team demonstrated the role of triple therapy in advanced BTC in a real-world study, which showing an ORR of 43.9%, median OS of 13.4 months, and median PFS of 9.27 months [18]. However, the study was only a single-center study, included both first-line treatment and non-first-line treatment for BTC patients, and fewer ICC patients were treated with triple therapy as the first-line treatment (only 14 cases) [18]. These low sample size data cannot provide a detailed understanding of the exact efficacy of triple therapy as the first-line treatment for advanced ICC in a real world study.
Based on the above research results, we conducted a multicenter retrospective study to evaluate the efficacy, safety, and prognostic factors for survival of PD-1 inhibitors combined with lenvatinib and Gemox chemotherapy as first-line systemic therapy for patients with advanced ICC in a real-world study. We believe that PD-1 inhibitors plus lenvatinib and Gemox chemotherapy may be an exciting therapeutic regimen for patients with advanced ICC.
Materials and methods
Study design and population
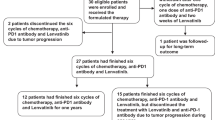
This multicenter retrospective study assessed the efficacy and safety of PD-1 inhibitors combined with lenvatinib plus Gemox chemotherapy as first-line therapy for advanced ICC at the Peking Union Medical College Hospital (PUMCH) and The Fifth Medical Center of PLA General Hospital (PLAGH). A total of 104 patients with advanced ICC who received a PD-1 inhibitor combined with lenvatinib and Gemox chemotherapy were enrolled in this study from June 2020 to September 2022. The primary eligibility criteria were histologically confirmed intrahepatic cholangiocarcinoma and at least one measurable tumor lesion according to the RECIST v1.1 criteria [19]. Among the initial 104 patients, 15 had combined use of one cycle, 19 did not receive triple combined regimens, 12 did not have measurable target lesions, and 5 had other additional malignant tumors (Fig. 1). Finally, 53 patients were enrolled in this study, 30 and 23 were enrolled in the PUMCH and PLAGH groups, respectively. Information on age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, Child–Pugh score, carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), hepatitis B virus (HBV) infection, maximum tumor diameter, tumor burden score (TBS), differentiated histology, tumor-node metastasis classification (TNM) stage, site of metastases, PD-L1 expression, and type of PD-1 inhibitors were compiled and recorded (Table 1). The TBS was calculated based on the maximum tumor size and the number of tumors in the liver [20, 21].
Treatment protocol
Information regarding the dates of initiation and completion of treatment, initial dose, radiological evaluation, laboratory data, and adverse events (AEs) during treatment was systematically collected. Lenvatinib was administered orally at a dose of 12 mg (for patients with body weight ≥ 60 kg) or 8 mg (for patients with body weight < 60 kg) once a day. Anti-PD-1 antibodies were administered at a fixed dose of 200 mg (240 mg for toripalimab) or a fixed dose of 3 mg/kg body weight every 3 weeks. The Gemox chemotherapy regimen was administered as 1 g/m2 of gemcitabine on days 1 and 8, 100 mg/m2 of oxaliplatin on day 1, and every 3 weeks by IV injection for six cycles.
Response assessment and safety evaluation
The clinical objective response was measured using the RECIST v1.1 criteria [19] and evaluated by professional radiologists at PUMCH and PLAGH. Computed tomography (CT) and magnetic resonance imaging (MRI) were performed to assess treatment response. The primary endpoints were OS and PFS, whereas the secondary endpoints were the ORR, disease control rate (DCR), clinical benefit rate (CBR) and safety. CBR was defined as the proportion of patients with a radiologically confirmed objective response (complete response [CR] or partial response [PR]) or stable disease (SD) for > 6 months [22]. Safety were recorded by physical examination, laboratory evaluation, and electronic medical records or collected by the investigators using the Common Terminology Criteria for Adverse Events (version 5.0) as a reference [23].
Evaluation of PD-L1 expression
Whole sections from formalin-fixed, paraffin-embedded tumor specimens were subjected to immunohistochemistry. For each tissue slice, 5-μm-thick sections were selected and placed on glass slides. The primary antibody used was anti-PD-L1, followed by the addition of secondary antibodies to all sections, including the negative control slides. Evaluation of PD-L1 expression was performed by independent pathologists who were blinded to the clinicopathological data, including therapeutic response and survival time. PD-L1 positivity or overexpression was defined as > 5% positive expression in tumor cells.
Statistical analysis
The cutoff date for analysis was December 30, 2022 in this study. Survival curves were estimated using the Kaplan–Meier method, and the log-rank test were used to analyzed the comparison groups. Hazard ratios (HRs) of each clinical factors for PFS and OS were estimated using the Cox proportional hazard model. For comparisons of individual variables, the t-test, Mann–Whitney U test, χ2 test, and Fisher’s exact test were performed as appropriate. Results with two-tailed p-values < 0.05 were considered statistically significant. Statistical analyses were performed using R-4.2.0 (https://www.r-project.org/) and the SPSS 25 software.
Results
Baseline characteristics
We screened 104 patients with advanced ICC who were treated in PUMCH and PLAGH from June 2020 to September 2022; 51 patients were excluded from the study (Fig. 1). Finally, we included 53 patients with advanced ICC who received PD-1 inhibitors combined with lenvatinib and Gemox chemotherapy as first-line treatment. The demographic and baseline characteristics of the 53 patients are summarized in Table 1. At the time of initial treatment, the median age was 58 years, with 41.5% of the patients being over 60 years old and 37.7% being women. We observed that 28 (52.8%) patients had an ECOG performance status of 0, and 32 (60.4%) patients had Child–Pugh score A. At baseline, the median CA19-9 level was 210 U/mL; 56.6% of the patients had a level > 200 U/mL. The median CEA level was 4.2 ng/mL; 41.5% of the patients had a level > 5 ng/mL. We observed that 10 patients (18.9%) had a history of HBV infection. The median maximum tumor diameter was 5.4 cm, and 54.7% of the patients had a level > 5 cm. At baseline, 22 patients (41.5%) had TBS > 8. In total, 17 (32.1%) patients had poorly differentiated histology, 23 (43.4%) had TNM stage III, and 17 (32.1%) had positive PD-L1 expression. Further, we observed that before treatment, most patients had metastatic tumors in the liver (36/53, 67.9%), lymph nodes (34/53, 64.2%), lungs (7/53, 13.2%), and bones (4/53, 7.5%). Among the 53 patients who had received different types of PD-1 inhibitors, 29 (54.7%) were treated with the toripalimab regimen, 11 (20.8%) with the tislelizumab regimen, 7 (13.2%) with the camrelizumab regimen, and 6 (11.3%) with the pembrolizumab regimen.
Treatment and efficacy
The median duration of treatment with PD-1 inhibitors combined with lenvatinib and Gemox chemotherapy was 8.07 (interquartile range: 5.3–11.6) months. The treatment duration for all the patients is shown in Fig. 2a. The median follow-up time was 13.7 (95% confidence interval (CI): 12.9–17.2) months for all participants in our cohort. All patients underwent a complete radiological evaluation. Overall, we identified 36 (67.9%) patients with decreased tumor sizes from baseline (Fig. 2b). Interestingly, we observed that 28 (52.8%) patients achieved an objective response, including 3 (5.7%) who showed CR and 25 (47.1%) who showed PR. We observed that 22 (41.5%) patients exhibited SD, whereas 3 (5.7%) exhibited PD. Consistently, we observed that the overall radiologically confirmed ORR was 52.8% (95% CI: 39.7–65.6%), and DCR was 94.3% (95% CI: 84.6–98.1%) (Fig. 2b, Table 2).
Therapeutic efficacy of PD-1 inhibitors combined with lenvatinib plus Gemox chemotherapy in patients with advanced intrahepatic cholangiocarcinoma. Treatment duration (a). Maximum percentage change in the sum of the diameters of the target lesions from baseline (b). Kaplan–Meier estimation of overall survival (c) and progression-free survival (d) in the entire cohort. *The first response was defined as the first time assessed as partial or complete response
We investigated the survival outcomes of the enrolled patients. For the entire cohort, we observed that the median OS was 14.3 (95% CI: 11.3–NR) and the median PFS was 8.63 (95% CI: 7.17–11.6) months (Fig. 2c, d, Table 2). The 6 months and 12 months OS were 90.0% (95% CI: 82.1–98.7%) and 59.6% (95% CI: 46.6–76.3%), respectively (Table 2). The 6 months and 12 months PFS were 73.3% (95% CI: 61.8–86.9%) and 31.3% (95% CI: 19.6–49.9%), respectively (Table 2). We further determined CBR in all patients. We observed that the CBR in all 53 patients was 75.5% (95% CI: 62.4–85.1%) (Fig. 2b, Table 2).
Six patients underwent conversion surgery after triple combination treatment, with a patient ID of 16, 22, 31, 41, 47, and 50, respectively (Fig. 2a, Table S1). Four patients achieved PR, and two achieved CR before conversion surgery. A median of 5 cycles of triple therapy was administered before conversion surgery (Table S1). One patient (patient ID 16) had disease progression 1.2 months after undergoing conversion surgery and changed treatment regimens. The remaining 5 patients had no disease progression at the time of the last follow-up and were receiving maintenance therapy with a PD-1 inhibitor plus lenvatinib.
Subgroup analyses and prognostic factors
Twelve potential prognostic variables for PFS and OS were first selected using univariate Cox analysis, including age, sex, ECOG PS, Child–Pugh score, CA19-9, CEA, HBV infection, maximum tumor diameter, TBS, differentiated histology, TNM stage, and PD-L1 expression (Table 3). In the univariate Cox analysis, TBS (≥ 8 vs. < 8; HR: 3.5; 95% CI: 1.6–7.4; P = 0.001), TNM stage (IV vs. III; HR: 4; 95% CI: 1.8–8.8; P < 0.001), and PD-L1 expression (positive vs. negative; HR: 0.18; 95% CI: 0.071–0.44; P < 0.001) were different for PFS, and ECOG PS (0 vs. ≥ 1; HR: 0.33; 95% CI: 0.15–0.74, P = 0.007), CA19-9 (≥ 200 vs. < 200; HR: 3.8; 95% CI: 1.6–8.9; P = 0.003), TBS (≥ 8 vs. < 8; HR: 4.8; 95% CI: 2.1–11; P < 0.001), differentiated histology (poor vs. moderate + well; HR: 2.8; 95% CI: 1.1–6.7; P = 0.024), TNM stage (IV vs. III; HR: 2.6; 95% CI: 1.1–6.1; P = 0.029), and PD-L1 expression (positive vs. negative; HR: 0.044; 95% CI: 0.0095–0.2; P < 0.001) were different for OS. Six factors that differed in the univariate Cox analysis, including ECOG PS, CA19-9, TBS, differentiated histology, TNM stage, and PD-L1 expression, were further subjected to multivariate Cox analysis. In the multivariate Cox analysis, TBS (≥ 8 vs. < 8; HR: 3.86; 95% CI: 1.32–11.29; P = 0.014), TNM stage (IV vs. III; HR: 6.69; 95% CI: 2.198–20.34; P < 0.001), and PD-L1 expression (positive vs. negative; HR: 0.27; 95% CI: 0.077–0.92; P = 0.037) were different for PFS, and TBS (≥ 8 vs. < 8; HR: 6.31; 95% CI: 1.659–24.0; P = 0.007) and PD-L1 expression (positive vs. negative; HR: 0.11; 95% CI: 0.018–0.7; P = 0.019) were different for OS (Table 3, Fig. 3a).
Subgroup analyses and prognostic factors. Subgroup analyses of progression-free survival (PFS) and overall survival (OS) in the entire cohort (a). Kaplan–Meier plots for PFS (b) and OS (c) based on TBS. Kaplan–Meier plots for PFS (d) and OS (e) based on the TNM stage. Kaplan–Meier plots for PFS (f) and OS (g) based on PD-L1 expression
Subgroup analyses of PFS and OS for three potentially prognostic variables, including TBS, TNM stage, and PD-L1 expression, were performed in the entire cohort. When we stratified patients according to TBS, the Kaplan–Meier survival curve and log-rank test analysis demonstrated that patients with TBS < 8 had a longer median PFS (10.7 vs. 6.6 months, P < 0.001; Fig. 3b) and a longer median OS (16.93 vs. 9.07 months, P < 0.001; Fig. 3c) than those with TBS ≥ 8. When we stratified patients according to the TNM stage, the Kaplan–Meier survival curve and log-rank test analysis showed that patients with TNM stage III had a longer median PFS (13.17 vs. 7.17 months, P < 0.001; Fig. 3d) and a longer median OS (16.9 vs. 11.3 months, P = 0.024; Fig. 3e) than those with TNM stage IV. When we stratified patients according to PD-L1 expression, the Kaplan–Meier survival curve and log-rank test analysis revealed that patients with positive PD-L1 expression had a longer median PFS (13.17 vs. 6.87 months, P < 0.001; Fig. 3f) and a longer median OS (NR vs. 9.57 months, P < 0.001; Fig. 3g) than those with negative PD-L1 expression.
Tolerability and safety
AEs were reported in all 53 patients (100%) throughout the study (Table S4, Fig. 4a). However, we did not detect grade 5 AE. Regarding severe AEs (SAEs), 41.5% (22/53) of the patients had ≥ grade 3 AEs, and only 1.9% (1/53) experienced grade 4 AEs (myelosuppression). The most common AEs (of any grade) were fatigue (31/53, 58.5%), myelosuppression (14/53, 26.4%), and decreased appetite (12/53, 22.6%). Most AEs that occurred during combination immunotherapy were not fatal, well-tolerated, and controlled. Particularly, the most common grade 3 or 4 SAEs were fatigue (8/53, 15.1%), myelosuppression (7/53, 13.2%), abdominal pain (4/53, 7.5%), hypertension (4/53, 7.5%), and bilirubin elevation (4/53, 7.5%).
We compared the AEs that occurred in the different PD-1 inhibitor groups, and there was no statistically significant difference in grade 3–4 AEs between the different PD-1 inhibitor groups (Table S2, Fig. 4b). In the toripalimab group, the most common grade 3–4 AEs were fatigue (17.2%, 5/29) and bilirubin elevation (10.3%, 3/29). In the tislelizumab group, the most common grade 3–4 AEs were fatigue (27.3%, 3/11), myelosuppression (18.2%, 2/11), and hypertension (18.2%, 2/11). In the camrelizumab group, the most common grade 3–4 AEs were myelosuppression (14.3%, 1/7), decreased appetite (14.3%, 1/7), and skin rashes (14.3%, 1/7). In the pembrolizumab group, the most common grade 3–4 AEs were myelosuppression (33.3%, 2/6) and abdominal pain (33.3%, 2/6). After careful treatment, we discovered that all observed AEs were controllable.
Discussion
To our knowledge, this is the first, largest sample size and multicenter study to investigate PD-1 inhibitors plus lenvatinib with Gemox chemotherapy as the first-line treatment option for advanced ICC in a real-world study. In this study, the triple combination regimens showed good efficacy and tolerable adverse reactions, with a median OS of 14.3, a median PFS of 8.63 months, and an ORR of 52.8%. Subgroup analysis confirmed three potential prognostic variables: TBS, TNM stage, and PD-L1 expression for PFS and OS. The rate of grade 3 and 4 AEs was 41.5% (22/53), which is acceptable, tolerable, and controllable.
PD-1 inhibitors, which are important components of ICIs, are increasingly used in BTC therapy [8, 24, 25]. Nivolumab combined with gemcitabine and tegafur chemotherapy has shown a good therapeutic effect in the first-line treatment of advanced BTC, with an ORR of 41.7% [24]. A study of PD-1 inhibitors plus lenvatinib for unresectable BTC showed an ORR of 42.1% [8]. These findings suggest that a combination of drugs with different mechanisms of action can overcome or improve the drug resistance of single-drug applications. Some studies suggest that chemotherapy may enhance the efficacy of PD-1 inhibitors through the following mechanisms: suppression of antitumor immunity by reducing myeloid-derived suppressor cells, selectively depleting monocytes/macrophages, enhancing the recruitment of antigen-presenting cells, and promoting the phagocytosis of dendritic cells through cytokines produced by cytotoxic chemotherapy damage to cancer cells [11,12,13]. Lenvatinib can promote the efficacy of immunotherapy by eliminating cancer cells through direct antitumor activity and immunogenic cell death and by reducing the number of cells targeted and destroyed by immune cells [26, 27].
Two clinical trials by Zhou et al. and Li et al. confirmed the efficacy of PD-1 inhibitors combined with lenvatinib and Gemox chemotherapy in ICC or BTC [16, 17]. When these two studies were compared with the current study, they consistently showed high ORRs despite using different PD-1 inhibitors and study endpoints (Table S3). The ORRs obtained in this study, the study by Zhou et al., and the study by Li were 52.8, 80, and 56%, respectively. The primary endpoint of the study by Li et al. was R0 resection rate (52%), with the major eligibility criteria being potentially resectable locally advanced BTC. In this study, 6 patients successfully underwent conversion surgery, suggesting that triple combined therapy regimens may be an option for patients with potential conversion surgery (Table S1, Figure S1). Multiple PD-1 inhibitors were used in our study compared to two reported clinical trials [16, 17]. Several other studies have reported that different types of PD-1 inhibitors have positive effects [8, 10, 22, 24, 25, 28]. We also performed subgroup analyses for different anti-PD-1 antibody regimens. We discovered that no significant differences were observed in the median PFS (9.90 vs. 7.55 vs. 7.62 vs. 9.77 months, P = 0.41; Figure S2A) and the median OS (11.6 vs. 13.5 vs. 11.3 vs. 15.6 months, P = 0.34; Figure S2B) among the camrelizumab, pembrolizumab, tislelizumab, and toripalimab groups. We performed subgroup analyses in the non-toripalimab and toripalimab groups and found no significant differences in the median PFS (8.0 vs. 9.77 months, P = 0.13; Figure S2C) and the median OS (11.6 vs. 15.6 months, P = 0.39, Figure S2D).
In this study, although each patient experienced varying degrees of AE, the incidence of severe AE was not significantly higher than that in other studies. Myelosuppression is a common AE of chemotherapy [3, 29]. In this study, 26.4% (14/53) of patients had varying degrees of myelosuppression, of which 11.3% (6/53) had grade 3–4 AE. In some studies on different combinations of PD-1 inhibitors, chemotherapy, and targeted therapy, the incidence of grade 3–4 AE was as high as 59.5% [3, 7, 29]. However, the incidence of grade 3–4 AE in our study was 41.5% (22/53), which is not higher than that reported in previous studies. In this study, no grade 5 AEs occurred, suggesting that PD-1 inhibitor plus lenvatinib with Gemox chemotherapy did not impose an additional burden on patients with AEs in the context of good efficacy.
This study has some limitations. First, although this was a multicenter real-world study, the total sample size was still limited due to the selection of treatment regimens and the incidence of diseases. In the future, multicenter cohort studies with larger sample sizes are needed to investigate the efficacy and tolerability of triple combined regimens. Second, multiple PD-1 inhibitors were administered in this study. Although there was no significant difference in survival and AE in the subgroup analysis, there may be a certain bias due to the small sample size of some PD-1 inhibitors. Future studies with single PD-1 inhibitors and large sample sizes are needed to verify whether there are differences among different PD-1 inhibitors. Third, this study lacks a cohort of standard chemotherapy-based regimens as controls, and prospective cohort study designs are needed to compensate for this deficiency in the future. Finally, although three potential prognostic variables were confirmed in this study, we were unable to collect and analyze more potential factors, such as the tumor mutational burden. Thus, future studies that include more prognostic factors should be conducted. Nonetheless, the results of this study can be used as a reference for the design of subsequent clinical studies and the selection of clinical treatment strategies.
In conclusion, PD-1 inhibitors combined with lenvatinib and Gemox chemotherapy are effective, safe, and well-tolerated as first-line therapies for advanced ICC. In addition, TBS, TNM stage, and PD-L1 expression can be used as potential prognostic factors.
Data availability statement
Data are available upon reasonable request.
Abbreviations
- AEs:
-
Adverse events
- BTC:
-
Biliary tract cancers
- CA19-9:
-
Carbohydrate antigen 19-9
- CEA:
-
Carcinoembryonic antigen
- CT:
-
Computed tomography
- CI:
-
Confidence interval
- CBR:
-
Clinical benefit rate
- CR:
-
Complete response
- DCR:
-
Disease control rate
- ECOG:
-
Eastern Cooperative Oncology Group
- FOLFOX:
-
Folinic acid, fluorouracil, and oxaliplatin
- Gemox:
-
Oxaliplatin, and gemcitabine
- GC:
-
Gemcitabine plus cisplatin
- HBV:
-
Hepatitis B virus
- HR:
-
Hazard ratios
- ICIs:
-
Immune checkpoint inhibitors
- ICC:
-
Intrahepatic cholangiocarcinoma
- MRI:
-
Magnetic resonance imaging
- OS:
-
Overall survival
- ORR:
-
Objective response rate
- PUMCH:
-
Peking Union Medical College Hospital
- PLAGH:
-
The Fifth Medical Center of PLA General Hospital
- PD-1:
-
Programmed cell death protein-1
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- SD:
-
Stable disease
- TBS:
-
Tumor burden score
- TNM:
-
Tumor-node metastasis classification
- VEGFR:
-
Vascular endothelial growth factor receptors
References
Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX (2021) Biliary tract cancer. Lancet 397:428–444. https://doi.org/10.1016/S0140-6736(21)00153-7
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A et al (2010) Cisplatin plus gemcitabine versus gemcitabine for Biliary Tract Cancer. N Engl J Med 362:1273–1281. https://doi.org/10.1056/NEJMoa0908721
Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A et al (2021) Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol 22:690–701. https://doi.org/10.1016/S1470-2045(21)00027-9
Chen X, Wu X, Wu H, Gu Y, Shao Y, Shao Q et al (2020) Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial. J Immunother Cancer 8:e001240. https://doi.org/10.1136/jitc-2020-001240
Feng K, Liu Y, Zhao Y, Yang Q, Dong L, Liu J et al (2020) Efficacy and biomarker analysis of nivolumab plus gemcitabine and cisplatin in patients with unresectable or metastatic biliary tract cancers: Results from a phase II study. J Immunother Cancer 8:e000367. https://doi.org/10.1136/jitc-2019-000367
Lin J, Yang X, Long J, Zhao S, Mao J, Wang D et al (2020) Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg Nutr 9:414–424. https://doi.org/10.21037/hbsn-20-338
Zhang Q, Liu X, Wei S, Zhang L, Tian Y, Gao Z et al (2021) Lenvatinib plus PD-1 inhibitors as first-line treatment in patients with unresectable biliary tract cancer: a single-arm, open-label, Phase II study. Front Oncol 11:751391. https://doi.org/10.3389/fonc.2021.751391
Dreikhausen L, Kusnik A, Schulte N, Eckardt M, Teufel A, Gaiser T et al (2021) Durable response with lenvatinib and pembrolizumab combination therapy in a patient with pre-treated metastatic cholangiocarcinoma. J Gastrointestin Liver Dis. https://doi.org/10.15403/jgld-3730
Shi C, Li Y, Yang C, Qiao L, Tang L, Zheng Y et al (2022) Lenvatinib plus programmed cell death protein-1 inhibitor beyond first-line systemic therapy in refractory advanced biliary tract cancer: a real-world retrospective study in China. Front Immunol 13:946861. https://doi.org/10.3389/fimmu.2022.946861
Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D et al (2021) Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol 14:156. https://doi.org/10.1186/s13045-021-01164-5
Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G (2015) Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28:690–714. https://doi.org/10.1016/j.ccell.2015.10.012
Sun W, Patel A, Normolle D, Patel K, Ohr J, Lee JJ et al (2019) A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 125:902–909. https://doi.org/10.1002/cncr.31872
Ziogas AC, Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Terpos E et al (2012) VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer 130:857–864. https://doi.org/10.1002/ijc.26094
Basu A, Hoerning A, Datta D, Edelbauer M, Stack MP, Calzadilla K et al (2010) Cutting edge: Vascular endothelial growth factor-mediated signaling in human CD45RO+ CD4+ T cells promotes Akt and ERK activation and costimulates IFN-gamma production. J Immunol 184:545–549. https://doi.org/10.4049/jimmunol.0900397
Li H (2022) A single-arm, open-label, phase II study of tislelizumab combined with lenvatinib and Gemox regimen for conversion therapy of potentially resectable locally advanced biliary tract cancers. Ann Oncol 33:S570. https://doi.org/10.1016/j.annonc.2022.07.093
Jian Z, Fan J, Shi G-M, Huang X-Y, Wu D, Yang G-H et al (2021) Gemox chemotherapy in combination with anti-PD1 antibody toripalimab and lenvatinib as first-line treatment for advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. J Clin Oncol 39:4094. https://doi.org/10.1200/JCO.2021.39.15_suppl.4094
Zhu C, Xue J, Wang Y, Wang S, Zhang N, Wang Y et al (2023) Efficacy and safety of lenvatinib combined with PD-1/PD-L1 inhibitors plus Gemox chemotherapy in advanced biliary tract cancer. Front Immunol 14:1109292. https://doi.org/10.3389/fimmu.2023.1109292
Schwartz LH, Seymour L, Litière S, Ford R, Gwyther S, Mandrekar S et al (2016) RECIST 1.1—Standardisation and disease-specific adaptations: perspectives from the RECIST Working Group. Eur J Cancer 62:138–145. https://doi.org/10.1016/j.ejca.2016.03.082
Sasaki K, Morioka D, Conci S, Margonis GA, Sawada Y, Ruzzenente A et al (2018) The tumor burden score: a new “metro-ticket” prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg 267:132–141. https://doi.org/10.1097/SLA.0000000000002064
Vitale A, Lai Q, Farinati F, Bucci L, Giannini EG, Napoli L et al (2018) Utility of tumor burden score to stratify prognosis of patients with hepatocellular cancer: results of 4759 cases from ITA.LI.CA Study Group. ITA.LI.CA. J Gastrointest Surg 22:859–871. https://doi.org/10.1007/s11605-018-3688-y
Quispel-Janssen J, van der Noort V, de Vries JF, Zimmerman M, Lalezari F, Thunnissen E et al (2018) Programmed death 1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol 13:1569–1576. https://doi.org/10.1016/j.jtho.2018.05.038
Freites-Martinez A, Santana N, Arias-Santiago S, Viera A (2021) Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermo Sifiliogr 112:90–92. https://doi.org/10.1016/j.ad.2019.05.009
Chiang N, Bai L, Huang C, Chen S, Hsiao C, Shan Y et al (2021) 49P A phase II trial of nivolumab and gemcitabine and S-1 as the first-line treatment in patients with advanced biliary tract cancer. Ann Oncol 32:S376–S381. https://doi.org/10.1016/j.annonc.2021.08.328
Villanueva L, Lwin Z, Chung HCC, Gomez-Roca CA, Longo F, Yanez E et al (2021) Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase 2 LEAP-005 study. J Clin Oncol 39:4080. https://doi.org/10.1200/JCO.2021.39.15_suppl.4080
Patel SA, Minn AJ (2018) Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity 48:417–433. https://doi.org/10.1016/j.immuni.2018.03.007
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P et al (2018) Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ 25:486–541. https://doi.org/10.1038/s41418-017-0012-4
Ueno M, Ikeda M, Morizane C, Kobayashi S, Ohno I, Kondo S et al (2019) Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol 4:611–621. https://doi.org/10.1016/S2468-1253(19)30086-X
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11:121–128. https://doi.org/10.1016/S1470-2045(09)70364-X
Funding
This work was supported by National High Level Hospital Clinical Research Funding [2022-PUMCH-B-128], CAMS Innovation Fund for Medical Sciences (CIFMS) [2022-I2M-C&T-A-003] [2021-I2M-1-061] [2021-I2M-1-003], CSCO-hengrui Cancer Research Fund [Y-HR2019-0239] [Y-HR2020MS-0415] [Y-HR2020QN-0414], CSCO-MSD Cancer Research Fund [Y-MSDZD2021-0213] and National Ten-thousand Talent Program.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design and data collection. Material preparation and analysis was performed by Chengpei Zhu, Hu Li and Xiaobo Yang. Chengpei Zhu, Hu Li and Xiaobo Yang wrote the first draft of the manuscript and all authors commented on the subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by the Institutional Review Board and Ethics Committee of PUMCH (IRB No. JS-1391), and the Fifth Medical Center of PLAGH (IRB No. KY-2022-4-24-1). The protocol was conformed to Good Clinical Practice guidelines and Declaration of Helsinki principles. Informed consent was provided by patients or waived by the ethical review committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lay Summaries: PD-1 inhibitors, lenvatinib and Gemox chemotherapy are effective and well-tolerated as first-line treatment in advanced ICC. TBS, TNM stage, and PD-L1 expression can be used as potential prognostic factors for OS and PFS. PD-1 inhibitors, lenvatinib and chemotherapy have a synergistic anti-tumor effect.
Supplementary Information
Figure S1. A patient with conversion surgery
Computed tomography (CT) image at pretreatment (A) and before surgery (B). H&E staining of the resected specimen (C). (D) Resected specimen.
Figure S2. Kaplan–Meier plot for progression-free survival (PFS) (A) and overall survival (OS) (B) based on four different types of PD-1 inhibitor groups. Kaplan–Meier plot for PFS (C) and OS (D) based on the non-toripalimab and toripalimab groups
Table S1. Main information of the six patients who underwent conversion surgery after triple combination treatment.
Table S2. Adverse events of different types of PD-1 inhibitors.
Table S3. The inclusion criteria, baseline characteristics, study endpoint, and therapeutic response of the present study compared with two other studies.
Table S4. Commonly observed adverse events. Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, C., Li, H., Yang, X. et al. Efficacy, safety, and prognostic factors of PD-1 inhibitors combined with lenvatinib and Gemox chemotherapy as first-line treatment in advanced intrahepatic cholangiocarcinoma: a multicenter real-world study. Cancer Immunol Immunother 72, 2949–2960 (2023). https://doi.org/10.1007/s00262-023-03466-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03466-8