Abstract
Introduction
Immune checkpoint inhibitors (ICI) such as anti-PD-L1 and anti-PD-1 agents have been proven to be effective in various cancers. However, the rate of non-responders is still high in all cancer entities. Therefore, the identification of biomarkers that could help to optimize therapeutic decision-making is of great clinical importance. Soluble PD-L1 (sPD-L1) and PD-1 (sPD-1) are emerging blood-based biomarkers and were previously shown to be prognostic in various clinical studies.
Objective
We aimed to evaluate the prognostic relevance of sPD-L1 and sPD-1 in patients with different tumor entities who underwent ICI therapy.
Methods
We searched for articles in PubMed via Medline, Embase, Scopus, and Cochrane databases. The primary outcome was overall survival (OS) and progression-free survival (PFS); furthermore, we analyzed on-treatment serum level changes of sPD-L1 and sPD-1 during ICI therapy.
Results
We synthesized the data of 1,054 patients with different cancer types from 15 articles. Pooled univariate analysis showed that elevated levels of sPD-L1 were significantly associated with inferior OS (HR = 1.67; CI:1.26–2.23, I2 = 79%, p < 0.001). The strongest association was found in non-small cell lung cancer, whereas weaker or no association was observed in melanoma as well as in renal cell and esophageal cancers. Pooled multivariate analysis also showed that elevated levels of sPD-L1 correlated with worse OS (HR = 1.62; CI: 1.00–2.62, I2 = 84%, p = 0.05) and PFS (HR = 1.71; CI:1.00–2.94, I2 = 82%, p = 0.051). Furthermore, we observed that one or three months of anti-PD-L1 treatment caused a strong (27.67-fold) elevation of sPD-L1 levels in malignant mesothelioma and urothelial cancer.
Conclusions
We found significantly inferior OS in ICI-treated cancer patients with elevated pre-treatment sPD-L1 levels, but this association seems to be tumor type dependent. In addition, sPD-L1 increases during anti-PD-L1 therapy seems to be therapy specific.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, growing evidence suggests that immune checkpoint inhibition with both programmed death protein-1 (PD-1) and programmed death protein ligand-1 (PD-L1) inhibitors is effective therapeutic options for several cancer types. Immune checkpoint inhibitors (ICI) revolutionized anti-cancer therapy, but the rate of non-responders is still high and varies significantly between various cancers [1,2,3,4]. Therefore, there is a great clinical need for prognostic and predictive biomarkers to identify patients who will respond to ICI therapy. Currently, ICIs are widely used in non-small cell lung cancer (NSCLC), melanoma, renal cell carcinoma (RCC), urothelial cancer, and breast cancer, but the list of indications is rapidly expanding [5].
PD-1 and PD-L1 are membrane-bound co-inhibitory immune checkpoint receptors expressed by various human immune and cancer cells. PD-1 is primarily located in T-cells, whereas PD-L1 is most abundantly expressed by cancer cells. Binding between PD-L1 and its receptor PD-1 leads to immune suppression, which helps cancer cells to escape from cytotoxic T-cell-mediated lysis [6]. PD-1 and PD-L1 are not only found on the surface of cells, but their soluble forms can be detected in blood circulation both in healthy individuals and cancer patients. Similar to its membrane-bond tissue expression, elevated serum PD-L1 (sPD-L1) and PD-1 (sPD-1) levels were generally associated with more advanced disease stages and worse survival, suggesting that these serum markers are prognostic in various tumors [7,8,9,10,11]. However, their predictive value regarding various systemic treatments remained largely contradictory.
There are only few available biomarkers for ICI therapy, such as tissue PD-L1 immunohistochemistry (IHC), tumor mutational burden, or microsatellite instability. However, their predictive ability is different among various tumor types.[12, 13]. For example, PD-L1 immunohistochemistry shows predictive value in NSCLC, head and neck squamous cell (HNSCC), and urothelial cancer [14,15,16], whereas in melanoma and RCC, PD-L1 IHC cannot be used for the prediction of ICI therapy [17, 18]. In addition, tissue-based IHC has further limitations related to the heterogeneity of PD-L1 expression, different characteristics of various diagnostic antibodies, and differences in evaluation methods [19]. Furthermore, as repeated biopsy for follow-up purposes is hardly feasible, tissue analysis is much less suitable for therapy monitoring than serum-based assays. Therefore, an unmet clinical need is the application of easily accessible, blood-based biomarkers determined by an easy-to-use and robust analytical method for pre-treatment prediction and monitoring of ICI therapy.
In the present study, we conducted a systematic review and meta-analysis of published literature data to assess the prognostic significance of circulating sPD-L1 and sPD-1 levels in pre-treatment and on-treatment samples of tumor patients who underwent ICI therapy.
Methods
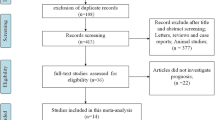
The study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 recommendations [20], and the Cochrane Handbook was followed [21]. The protocol was registered on PROSPERO (Nr.:CRD42021283222).
Literature search
Electronic databases from PubMed, Scopus, Embase, and Cochrane library were screened to identify studies investigating the prognostic role of sPD-L1 and sPD-1 in various cancers treated with ICIs. Additionally, references of included studies were screened to identify further potentially eligible studies. Two independent authors (AS and TF) performed the systematic search and the selection process. References were screened using EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA) and assessed by title, abstract, and full text.
Eligibility criteria
The PECO framework was applied to state our research question. We included original studies in the English language, which investigated (P) ICI-treated patients with various tumors, and (E and C) compared the hazard of high and low serum or plasma sPD-L1 and/or sPD-1 levels in regard to (O) overall survival (OS) or progression-free survival (PFS). There was no pre-defined cut-off value for the definition of high and low levels of biomarkers. If available, on-treatment sPD-L1 and sPD-1 concentrations (median or mean level, range, or interquartile range) were also considered as additionally assessed parameters.
The following exclusion criteria were used: study design: reviews, comments, letters, meta-analysis, systematic reviews, animal experiments, and conference abstracts. No restrictions were made regarding cohort size and study design.
Data extraction
Two independent authors (AS, TF) extracted data by reading full-text articles. Extracted parameters were the following: the first name of the author, year of publication, cancer type, ICI therapy type, country of sample/data collection, study type, cohort size, patient age, sex, cut-off values for sPD1/sPD-L1, cut-off definition method (e.g., median, receiver operating characteristic curve—ROC), assay method, follow-up time, OS, and PFS.
In eligible studies, either the article provided calculated hazard ratios (HR) with a 95% Confidence Interval (CI), or the overall HR and 95% CI were estimated from Kaplan–Meier curves by using the GetData Graph Digitizer software v2.26™. In addition, when available, data on changes of sPD-L1 and sPD-1 during ICI treatment were extracted.
Quality assessment and evaluation of evidence
Two independent authors performed the risk of bias assessment using the Quality in Prognostic Studies (QUIPS) tool [22]. The study attrition domain was assessed only in the case of prospective studies. We used the RobVisR tool to summarize the results of the assessments [23]. (Supplementary Table 1, Fig. 1) For the level of evidence assessment, the GRADEpro™ program [24]. (Supplementary Table 2) was applied.
Synthesis methods
Random-effects models with the inverse variance method were applied to pool hazard ratios (HR) with 95% confidence interval (CI) in the case of all outcomes. For the outcomes where the study number was over 5, a Hartung–Knapp adjustment [25, 26] was applied. The restricted maximum-likelihood method [27] was used to estimate variance measure 2τ2, and between-study heterogeneity was investigated with Cochrane Q test and the Higgins & Thompson’s I2I2 statistics [28]. The Q test was considered significant when the p value was less than 0.1. Forest plots were used to graphically summarize the results. Where applicable we reported the prediction intervals of results according to IntHout et al. [29]. Outlier and influence analyses were carried out following the recommendations of Harrer [27] and Viechtbauer and Cheung [30]. Small study effect was investigated on funnel plots, and if there had been at least 10 studies, it would have been assessed statistically using Egger’s test. Subgroup analysis was conducted based on the used ELISA assays and cancer types. In the case of subgroup analysis, a fixed-effects “plural” model was applied (aka. mixed-effects model). To assess the difference between the subgroups, Cochrane Q test was used [27]. The null hypothesis was rejected at a 5% significance level. All statistical analyses were performed with R [31] statistical environment and language, using the meta [32] and dmetar [33] packages. P < 0.05 was considered significant. Biomarker level changes were expressed as fold-changes, and a median fold-change was calculated separately for PD-1 and PD-L1 inhibitors (Table 2).
Results
Search and selection
Using the above-defined search key, 458 articles were initially retrieved from the accessed databases (Fig. 1). After the selection process, 16 articles matched our eligibility criteria [10, 34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. However, the HR and 95% CI estimation in two articles were not possible [34, 35]. Therefore, these two articles were included only in the qualitative synthesis.
Baseline characteristics of included studies
Baseline characteristics of the included articles are summarized in Table 1. Cancer types included in this systematic review were the following: NSCLC, RCC, melanoma, esophageal squamous cell cancer (ESCC), urothelial cancer, and mesothelioma. Nine articles reported the results of prospectively performed studies, and seven articles were retrospective. Only three articles included data on sPD-1, and all the included publications reported on sPD-L1. Different studies used different strategies to set cut-off values; six studies used the median as cut-off, while eight used the ROC analysis to adjust the cut-off values. All but one study used the ELISA assay technique to determine sPD-1 or sPD-L1 concentrations. Nine studies applied ELISA assays by R&D Systems (Wiesbaden, Germany), and other studies used assays by Cloud-Clone (Dynabio, Marseille, France), Abcam (Cambridge, UK), Invitrogen (Thermo Fisher, Darmstadt, Germany), and SIMOA (Billerica, MA, USA). The remaining article used a multiplex immunoassay (14-ProcartaPlex Human Immuno-Oncology Checkpoint Panel by Invitrogen (Thermo Fisher, Darmstadt, Germany)). Ten articles reported on-treatment sPD-1/sPD-L1 levels in addition to baseline levels [10, 34,35,36,37,38, 40, 42, 45, 47].
Elevated pre-treatment sPD-L1 predicts OS in NSCLC and melanoma
Thirteen articles reported univariate OS as a primary outcome. The pooled overall estimate showed that patients with high sPD-L1 levels had worse OS (HR:1.67; CI:1.26–2.23, I2 = 79%, p < 0.001; Fig. 2). As for publication bias, the funnel plot seems asymmetric; however, Egger’s test shows no publication bias (p = 0.177)(Supplementary Figs. 5 and 7).
Four of the included articles reported a multivariate Cox proportional hazard model. The pooled multivariate analysis confirmed that patients with high sPD-L1 levels had shorter OS (HR:1.62; CI:1.00–2.62, I2 = 84%, p = 0.05; Supplementary Fig. 2).
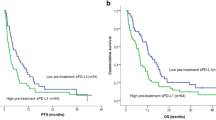
A subgroup analysis was performed according to cancer type. Based on six studies with NSCLC patients, high sPD-L1 levels were consequently associated with poor OS (HR:2.93; CI:2.52–3.40, I2 = 0%, p < 0.001). According to three publications, poorer OS was found for malignant melanoma (HR:1.73; CI:1.01–2.97, I2 = 19% p = 0.047). No difference was found between high and low sPD-L1 levels in OS in the subgroup of mixed tumor types (HR:1.22; CI:0.86–1.72, I2 = 0%, p = 0.263), but in this case, various studies showed rather heterogeneous results (Fig. 2).
Elevated pre-treatment sPD-L1 predicts poor PFS in NSCLC
Eleven articles reported univariate PFS as the primary outcome. The pooled overall estimate found no PFS difference between high and low sPD-L1 groups (HR:1.20; CI:0.85–1.70, I2 = 78%, p = 0.305; Fig. 3). The visual presentation of the Funnel plot and Egger’s test suggested publication bias (p = 0.007) (Supplementary Figs. 6 and 8).
Four of the included articles reported a multivariate Cox proportional hazard model. The pooled multivariate analysis showed that patients with high sPD-L1 levels tended to have inferior PFS (HR:1.71; CI:1.00–2.94, I2 = 82%, p = 0.051; Supplementary Fig. 3).
The subgroup analysis of cancer types revealed high pre-treatment sPD-L1 as a strong risk factor in the NSCLC subgroup (HR:2.08; CI:1.81–2.38, I2 = 0% p < 0.001), whereas rather heterogeneous results were observed in RCC (HR:0.67; CI:0.12–3.86, I2 = 88% p = 0.653), melanoma (HR:1.18; CI: 0.56–2.50, I2 = 74%, p = 0.668) and mixed cohorts (HR:0.96; CI:0.47–1.96, I2 = 74%, p = 0.903) (Fig. 3).
Pre-treatment sPD-1 and PFS and OS
Three articles reported PFS for sPD-1 (HR:1.16; CI:0.23–5.75, I2 = 89%, p = 0.858) (Supplementary Fig. 4) with heterogeneous results. Meyo et al. in NSCLC and Ugurel et al. found in melanoma that higher sPD-1 level patients had shorter PFS, whereas Incorvaia et al. found the opposite result in metastatic RCC [38, 42, 46].
Meyo et al. (HR:2.28; CI:1.11–4.68; p = 0.025) and Ugurel et al. (HR:2.70; CI:1.10–6.25; p = 0.055) reported sPD-1 and OS [42, 46].
sPD-L1 levels strongly increase during anti-PD-L1 therapy
Ten articles reported both pre-treatment and on-treatment sPD-L1 levels in 12 tumor entities. Serum sPD-L1 levels remained unchanged under anti-PD-1 therapy, whereas anti-PD-L1 therapy caused a remarkable (27.67-fold) elevation of sPD-L1 levels (Table 2). Two articles reported both pre-treatment and on-treatment sPD-1 levels during anti-PD-1 (nivolumab) therapy [38, 42] (Table 2).
The assay method does not seem to influence the correlations between sPD-L1 and OS
Our subgroup analysis according to the used assay methods suggested that the sPD-L1 assay method had no major influence on the OS (R&D: HR:2.11; CI:1.44–3.08, I2 = 84%, p = 0.003 vs. “others”: HR:1.35; CI:0.79–2.30, I2 = 54%, p = 0.224; Fig. 4). The same subgroup analysis was further evaluated based on PFS. Our subgroup analysis suggested that the sPD-L1 assay method might influence PFS. (R&D: HR:1.87; CI:1.52–2.32, I2 = 2%, p = 0.025 vs. “others”: HR:0.96; CI:0.55–1.66, I2 = 76%, p = 0.873; Fig. 5). Because of the low number of studies with sPD-1, no comparison was possible between various assay methods.
Risk of bias assessment and level of evidence
Based on author judgment, 12 out of 16 articles had a low risk of bias, while four carried a moderate risk (Supplementary Fig. 1, Supplementary Table 1).
Grading
On the basis of GRADEpro™, moderate certainty of the evidence was found for the two primary endpoints (Supplementary Table 2).
Discussion
This meta-analysis aimed to summarize the data of currently available literature on the prognostic significance of sPD-L1 and sPD-1 in various cancers in the aspect of ICI therapy. Serum sPD-1 and sPD-L1 are easily accessible biomarkers that may help in pre-treatment prognostication and in therapy monitoring of patients who underwent ICI therapy.
In the past few years, several studies assessed the association between sPD-L1 and prognosis in various cancers and treatment settings. Huang et al. constructed a meta-analysis in 2021 to assess the correlation between sPD-L1 and survival in a wide range of human malignancies [49]. The pooled overall estimate showed sPD-L1 as a significant indicator of shorter OS in various cancers. However, the article contained only three ICI therapy-related articles that did not allow to draw firm conclusions. Recently, a significant number of research articles have been published focusing on sPD-L1 (or sPD-1) levels in the context of ICI therapy, and these articles provided contradictory results concerning the prognostic role of sPD-L1. For example, Incorvaia et al. found that nivolumab-treated metastatic RCC patients with high sPD-L1, sPD-1, and BTN3A1 levels had better PFS [38]. In contrast, Mahoney et al. in the Checkmate 009 trial found no significant survival benefits for RCC patients with high sPD-L1 levels [40]. In addition, Ji et al. also found significantly higher disease control in ICI-treated ESCC patients as well as better survival rates for patients with high sPD-L1 levels [39]. In contrast, in NSCLC studies, high sPD-L1 levels consequently tended to be associated with shorter patient survival in ICI-treated patients. This finding is in line with the previous meta-analysis by Liao et al., suggesting that low rather than high sPD-L1 levels might have predictive values for ICI treatment [50].
In the present meta-analysis, summarizing data from 16 publications including more than six cancer types and an overall number of 1,054 ICI-treated patients, a 67% higher risk of death was found in patients with high sPD-L1 levels. Similarly, patients with high sPD-L1 levels had a 20% higher risk of disease progression. Interestingly, our subgroup analyses for different tumor entities revealed a heterogeneous pattern. For melanoma, we found three eligible publications with 198 ICI-treated patients, and the observed hazard ratios for OS and PFS revealed a rather heterogeneous picture regarding the prognostic value of sPD-L1 in melanoma. In contrast, for NSCLC, the pooled analysis of six studies with an overall number of 457 ICI-treated patients provided a much more consistent results for both OS and PFS across various studies. Overall, our summary suggests an association between higher sPD-L1 levels and poor prognosis. However, this effect may be different in distinct tumor types. On the basis of these, a tumor type-specific interpretation is suggested for the prognostic value of sPD-L1 in ICI-treated patients. The prognostic value of sPD-L1 is not sufficiently confirmed in melanoma patients, whereas several independent studies confirmed it in NSCLC. Therefore, in NSCLC, pre-treatment sPD-L1 may be a potential biomarker to predict OS and PFS before ICI therapy. On the other hand, in NSCLC, sPD-L1 levels were associated with shorter survival in other therapy settings, suggesting that sPD-L1 might be rather a prognostic than a predictive factor. Further prospective studies are necessary to address this question.
As there are several commercially available sPD-L1 assay kits, we assessed the potential influence of the assay method on study results. Overall, in the 16 included studies, seven different assay kits were applied, with the R&D kit as the most commonly used. However, slightly worse survival rates were found in studies that used the R&D assay, but the visual interpretation of the plot (Figs. 4 and 5) revealed a similar distribution of the articles around the line of no effect. Therefore, we conclude that the ELISA method may not significantly influence the outcomes.
Four articles presented the multivariate analysis of OS and PFS, and the pooled estimate showed high sPD-L1 as an independent risk factor for ICI therapy. However, these articles presented different factors as independent determinators of both OS and PFS. In these four articles, ECOG performance status was consequently found to be a significant independent predictor of survival, whereas tissue expression of PD-L1 was independently associated with poor OS in three articles. Furthermore, two articles showed high neutrophil-to-lymphocyte ratio as an independent predictor of poor OS in a multivariate analysis, suggesting that inflammation status has an inevitable impact on ICI-sensitivity.
Comparison between baseline and on-treatment sPD-L1 levels was possible in 12 studies. Based on our previous observation in urothelial cancer, we hypothesized that anti-PD-L1 therapy leads to an elevation in sPD-L1 levels [10]. Accordingly, in the two studies with presenting patients who received anti-PD-L1 therapy a strong (27- and 28-fold) increase in sPD-L1 levels could be observed [10, 36], whereas no such difference was detected in anti-PD-1-treated patients [34, 35, 37, 38, 40, 42, 45, 47]. Furthermore, sPD-1 levels did not increase after anti-PD-1 (nivolumab) therapy [38, 42]. In contrast, Music et al. observed that sPD-1 elevated after the administration of anti-PD-1 pembrolizumab therapy [51]. Therefore, it appears that anti-PD-L1 rather than anti-PD-1 therapy induces a significant increase in sPD-L1 levels. However, one possible explanation could be that ICIs—especially atezolizumab—can trigger a strong anti-drug-antibody (ADA) production, which may form antibody complexes that can enhance the measured ELISA signal [52]. On the basis of these, the on-treatment flare-up of sPD-L1 seems to be therapy specific for anti-PD-L1 therapy, but the biological and clinical relevance of this elevation needs to be further evaluated.
Our study has some limitations mainly related to the heterogeneity of the included studies regarding their cohort sizes, tumor types, applied ICI drugs, and cut-off values. A further limitation is the unavailability of radiographic response data.
The strength of our study is that it is the first meta-analysis focusing on the prognostic values of sPD-L1 and sPD-1 in a particular group of ICI-treated cancer patients. Furthermore, we evaluated 16 eligible studies with > 1,000 cases using both OS and PFS as endpoints and evaluated results in the context of tumor type, assay method, and marker level changes.
Conclusion
In conclusion, we found significantly worse OS in ICI-treated cancer patients with high baseline sPD-L1 levels, but this association seems to be tumor type dependent. Therefore, we suggest that sPD-L1 as a pre-treatment prognostic biomarker for ICI therapy, which should be interpreted in a tumor type-specific context. In addition, we found a remarkably strong increase in sPD-L1 during anti-PD-L1 treatment. The biological background and clinical significance of this sPD-L1 flare need to be evaluated in future studies. A further prospectively designed biomarker-based randomized clinical trial is of great need to reveal the therapy predictive role of sPD-L1.
References
Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L, Melero I, Schalper KA, Herbst RS (2019) Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res 25:4592–4602. https://doi.org/10.1158/1078-0432.CCR-18-1538
Gide TN, Wilmott JS, Scolyer RA, Long GV (2018) Primary and Acquired resistance to immune checkpoint inhibitors in metastatic melanoma. Clin Cancer Res 24:1260–1270. https://doi.org/10.1158/1078-0432.CCR-17-2267
Raimondi A, Randon G, Sepe P, Claps M, Verzoni E, de Braud F, Procopio G (2019) The evaluation of response to immunotherapy in metastatic renal cell carcinoma: open challenges in the clinical practice. Int J Mol Sci. https://doi.org/10.3390/ijms20174263
Ramos JD, Yu EY (2016) Making urothelial carcinomas less immune to immunotherapy. Urol Oncol 34:534–537. https://doi.org/10.1016/j.urolonc.2016.10.007
Jacob JB, Jacob MK, Parajuli P (2021) Review of immune checkpoint inhibitors in immuno-oncology. Adv Pharmacol 91:111–139. https://doi.org/10.1016/bs.apha.2021.01.002
Sun C, Mezzadra R, Schumacher TN (2018) Regulation and function of the PD-L1 checkpoint. Immunity 48:434–452. https://doi.org/10.1016/j.immuni.2018.03.014
Chang B, Huang T, Wei H, Shen L, Zhu D, He W, Chen Q, Zhang H, Li Y, Huang R et al (2019) The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother 68:353–363. https://doi.org/10.1007/s00262-018-2271-4
Abu Hejleh T, Furqan M, Ballas Z, Clamon G (2019) The clinical significance of soluble PD-1 and PD-L1 in lung cancer. Crit Rev Oncol Hematol 143:148–152. https://doi.org/10.1016/j.critrevonc.2019.08.009
Hassen G, Kasar A, Jain N, Berry S, Dave J, Zouetr M, Priyanka Ganapathiraju VLN, Kurapati T, Oshai S, Saad M et al (2022) Programmed death-ligand 1 (PD-L1) positivity and factors associated with poor prognosis in patients with gastric cancer: an umbrella meta-analysis. Cureus 14:e23845. https://doi.org/10.7759/cureus.23845
Krafft U, Olah C, Reis H, Kesch C, Darr C, Grunwald V, Tschirdewahn S, Hadaschik B, Horvath O, Kenessey I et al (2021) High serum PD-L1 levels are associated with poor survival in urothelial cancer patients treated with chemotherapy and immune checkpoint inhibitor therapy. Cancers (Basel). https://doi.org/10.3390/cancers13112548
Bian B, Fanale D, Dusetti N, Roque J, Pastor S, Chretien AS, Incorvaia L, Russo A, Olive D, Iovanna J (2019) Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. Oncoimmunology 8:e1561120. https://doi.org/10.1080/2162402X.2018.1561120
Palmeri M, Mehnert J, Silk AW, Jabbour SK, Ganesan S, Popli P, Riedlinger G, Stephenson R, de Meritens AB, Leiser A et al (2022) Real-world application of tumor mutational burden-high (TMB-high) and microsatellite instability (MSI) confirms their utility as immunotherapy biomarkers. ESMO Open 7:100336. https://doi.org/10.1016/j.esmoop.2021.100336
Patel SP, Kurzrock R (2015) PD-L1 Expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 14:847–856. https://doi.org/10.1158/1535-7163.MCT-14-0983
Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Psyrri A, Basté N, Neupane P, Bratland Å et al (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394:1915–1928. https://doi.org/10.1016/s0140-6736(19)32591-7
Lantuejoul S, Sound-Tsao M, Cooper WA, Girard N, Hirsch FR, Roden AC, Lopez-Rios F, Jain D, Chou TY, Motoi N et al (2020) PD-L1 testing for lung cancer in 2019: perspective from the IASLC pathology committee. J Thorac Oncol 15:499–519. https://doi.org/10.1016/j.jtho.2019.12.107
Eckstein M, Cimadamore A, Hartmann A, Lopez-Beltran A, Cheng L, Scarpelli M, Montironi R, Gevaert T (2019) PD-L1 assessment in urothelial carcinoma: a practical approach. Ann Transl Med 7:690. https://doi.org/10.21037/atm.2019.10.24
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813. https://doi.org/10.1056/NEJMoa1510665
Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J, Yearley JH, Kefford RF, Thompson JF, Long GV et al (2015) PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res 28:245–253. https://doi.org/10.1111/pcmr.12340
Paver EC, Cooper WA, Colebatch AJ, Ferguson PM, Hill SK, Lum T, Shin JS, O’Toole S, Anderson L, Scolyer RA et al (2021) Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: a guide to immunohistochemistry implementation and interpretation. Pathology 53:141–156. https://doi.org/10.1016/j.pathol.2020.10.007
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2019) Cochrane handbook for systematic reviews of interventions. John Wiley & Sons, Hoboken
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C (2013) Assessing Bias in Studies of Prognostic Factors. Ann Intern Med 158:280–286
McGuinness LA, Higgins JPT (2021) Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 12:55–61. https://doi.org/10.1002/jrsm.1411
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H et al (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394. https://doi.org/10.1016/j.jclinepi.2010.04.026
Knapp G, Hartung J (2003) Improved tests for a random effects meta-regression with a single covariate. Stat Med 22:2693–2710. https://doi.org/10.1002/sim.1482
IntHout J, Ioannidis JP, Borm GF (2014) The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 14:25. https://doi.org/10.1186/1471-2288-14-25
Harrer, M., Pim Cuijpers, Furukawa Toshi A, and David D Ebert (2021) Doing Meta-Analysis With R: A Hands-On Guide. Boca Raton, FL; London: Chapman & Hall/CRC Press. 1st ed.
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
IntHout J, Ioannidis JP, Rovers MM, Goeman JJ (2016) Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6:e010247. https://doi.org/10.1136/bmjopen-2015-010247
Viechtbauer W, Cheung MW (2010) Outlier and influence diagnostics for meta-analysis. Res Synth Methods 1:112–125. https://doi.org/10.1002/jrsm.11
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Schwarzer, G (2022) Meta‐Analysis in R. Systematic Reviews in Health Research: Meta‐Analysis in Context :510–534
Harrer, M., Cuijpers, P., Furukawa, T.A., & Ebert, D.D. (2021). Doing Meta-Analysis with R: A Hands-On Guide. Boca Raton, FL and London: Chapman & Hall/CRC Press. ISBN 978-0-367-61007-4. http://dmetar.protectlab.org
Ando K, Hamada K, Watanabe M, Ohkuma R, Shida M, Onoue R, Kubota Y, Matsui H, Ishiguro T, Hirasawa Y et al (2019) Plasma levels of soluble PD-L1 correlate With tumor regression in patients with lung and gastric cancer treated with immune checkpoint inhibitors. Anticancer Res 39:5195–5201. https://doi.org/10.21873/anticanres.13716
Castello A, Rossi S, Toschi L, Mansi L, Lopci E (2020) Soluble PD-L1 in NSCLC patients treated with checkpoint inhibitors and its correlation with metabolic parameters. Cancers (Basel). https://doi.org/10.3390/cancers12061373
Chiarucci C, Cannito S, Daffina MG, Amato G, Giacobini G, Cutaia O, Lofiego MF, Fazio C, Giannarelli D, Danielli R et al (2020) Circulating levels of PD-L1 in mesothelioma patients from the NIBIT-MESO-1 study: correlation with survival. Cancers (Basel). https://doi.org/10.3390/cancers12020361
Costantini A, Julie C, Dumenil C, Helias-Rodzewicz Z, Tisserand J, Dumoulin J, Giraud V, Labrune S, Chinet T, Emile JF et al (2018) Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. Oncoimmunology 7:e1452581. https://doi.org/10.1080/2162402X.2018.1452581
Incorvaia L, Fanale D, Badalamenti G, Porta C, Olive D, De Luca I, Brando C, Rizzo M, Messina C, Rediti M et al (2020) Baseline plasma levels of soluble PD-1, PD-L1, and BTN3A1 predict response to nivolumab treatment in patients with metastatic renal cell carcinoma: a step toward a biomarker for therapeutic decisions. Oncoimmunology 9:1832348. https://doi.org/10.1080/2162402X.2020.1832348
Ji S, Chen H, Yang K, Zhang G, Mao B, Hu Y, Zhang H, Xu J (2020) Peripheral cytokine levels as predictive biomarkers of benefit from immune checkpoint inhibitors in cancer therapy. Biomed Pharmacother 129:110457. https://doi.org/10.1016/j.biopha.2020.110457
Mahoney KM, Ross-Macdonald P, Yuan L, Song L, Veras E, Wind-Rotolo M, McDermott DF, Stephen Hodi F, Choueiri TK, Freeman GJ (2022) Soluble PD-L1 as an early marker of progressive disease on nivolumab. J Immunother Cancer. https://doi.org/10.1136/jitc-2021-003527
Mazzaschi G, Minari R, Zecca A, Cavazzoni A, Ferri V, Mori C, Squadrilli A, Bordi P, Buti S, Bersanelli M et al (2020) Soluble PD-L1 and Circulating CD8+PD-1+ and NK cells enclose a prognostic and predictive immune effector score in immunotherapy treated NSCLC patients. Lung Cancer 148:1–11. https://doi.org/10.1016/j.lungcan.2020.07.028
Tiako Meyo M, Jouinot A, Giroux-Leprieur E, Fabre E, Wislez M, Alifano M, Leroy K, Boudou-Rouquette P, Tlemsani C, Khoudour N et al (2020) Predictive value of soluble PD-1, PD-L1, VEGFA, CD40 ligand and CD44 for nivolumab therapy in advanced non-small cell lung cancer: a case-control study. Cancers (Basel). https://doi.org/10.3390/cancers12020473
Murakami S, Shibaki R, Matsumoto Y, Yoshida T, Goto Y, Kanda S, Horinouchi H, Fujiwara Y, Yamamoto N, Ohe Y (2020) Association between serum level soluble programmed cell death ligand 1 and prognosis in patients with non-small cell lung cancer treated with anti-PD-1 antibody. Thorac Cancer 11:3585–3595. https://doi.org/10.1111/1759-7714.13721
Okuma Y, Wakui H, Utsumi H, Sagawa Y, Hosomi Y, Kuwano K, Homma S (2018) Soluble Programmed cell death ligand 1 as a novel biomarker for nivolumab therapy for non-small-cell lung cancer. Clin Lung Cancer 19:410–417. https://doi.org/10.1016/j.cllc.2018.04.014
Oh SY, Kim S, Keam B, Kim TM, Kim DW, Heo DS (2021) Soluble PD-L1 is a predictive and prognostic biomarker in advanced cancer patients who receive immune checkpoint blockade treatment. Sci Rep 11:19712. https://doi.org/10.1038/s41598-021-99311-y
Ugurel S, Schadendorf D, Horny K, Sucker A, Schramm S, Utikal J, Pfohler C, Herbst R, Schilling B, Blank C et al (2020) Elevated baseline serum PD-1 or PD-L1 predicts poor outcome of PD-1 inhibition therapy in metastatic melanoma. Ann Oncol 31:144–152. https://doi.org/10.1016/j.annonc.2019.09.005
Yang Q, Chen M, Gu J, Niu K, Zhao X, Zheng L, Xu Z, Yu Y, Li F, Meng L et al (2021) Novel biomarkers of dynamic blood PD-L1 expression for immune checkpoint inhibitors in advanced non-small-cell lung cancer patients. Front Immunol 12:665133. https://doi.org/10.3389/fimmu.2021.665133
Zhou J, Mahoney KM, Giobbie-Hurder A, Zhao F, Lee S, Liao X, Rodig S, Li J, Wu X, Butterfield LH et al (2017) Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res 5:480–492. https://doi.org/10.1158/2326-6066.CIR-16-0329
Huang P, Hu W, Zhu Y, Wu Y, Lin H (2020) The Prognostic Value of Circulating Soluble Programmed Death Ligand-1 in Cancers: A Meta-Analysis. Front Oncol 10:626932. https://doi.org/10.3389/fonc.2020.626932
Liao G, Zhao Z, Qian Y, Ling X, Chen S, Li X, Kong FS (2021) Prognostic Role of Soluble Programmed Death Ligand 1 in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front Oncol 11:774131. https://doi.org/10.3389/fonc.2021.774131
Music M, Iafolla MAJ, Ren AH, Soosaipillai A, Prassas I, Diamandis EP (2019) Serum PD-1 Is Elevated after Pembrolizumab Treatment but Has No Predictive Value. Mol Cancer Ther 18:1844–1851. https://doi.org/10.1158/1535-7163.MCT-19-0132
Wu B, Sternheim N, Agarwal P, Suchomel J, Vadhavkar S, Bruno R, Ballinger M, Bernaards CA, Chan P, Ruppel J et al (2022) Evaluation of atezolizumab immunogenicity: Clinical pharmacology (part 1). Clin Transl Sci 15:130–140. https://doi.org/10.1111/cts.13127
Acknowledgements
This study was supported by the K139059 grant of the Ministry for Innovation and Technology from the source of the National Research Development and Innovation Fund. T.S. was supported by a János Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00451/20/5) and by the New National Excellence Program (ÚNKP-21-5-SE-3). A.C. was supported by the New National Excellence Program (ÚNKP-21-3-II-SE-13).
Funding
Open access funding provided by Semmelweis University. Sponsors had no role in the design, data collection, analysis, interpretation, and manuscript preparation.
Author information
Authors and Affiliations
Contributions
Conceptualization was contributed by AS, FT, and SV; project administration was contributed by SV, PH, PN, and TS; methodology was contributed by SV, BH, AC, MV, and AV; formal analysis was contributed by AV, BH, AC, and PTK; writing—original draft, was contributed by AS, AV, SV, and ST; visualization was contributed by PTK, UK, VG, UH, and AV; data curation was contributed by UK, VG, UH, and VG; writing—review and editing, was contributed by AS, FT, SV, BH, AC, PTK, BH, TS, VG, and NP; supervision was contributed by PH, NP, TS, and BH; funding acquisition was contributed by PH, NP, and TS. All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
BH reports personal fees from AAA/Novartis, ABX, Bayer, LightPoint Medical, Inc., Janssen R&D, Bristol–Myers Squibb, and Astellas; research funding from Profound Medical, German Cancer Aid, German Research Foundation, Janssen R&D, Bristol–Myers Squibb, MSD, Pfizer, and Astellas; and travel fees from AstraZeneca, Janssen R&D, and Astellas; all outside the current manuscript. The other authors report none to declare.
Ethical approval
No ethical approval was required for this systematic review with meta-analysis, as all data were already published in peer-reviewed journals. Furthermore, no patients were involved in the design, conduct, or interpretation of our study. The datasets used in this study can be found in the full-text articles included in the systematic review and meta-analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Széles, Á., Fazekas, T., Váncsa, S. et al. Pre-treatment soluble PD-L1 as a predictor of overall survival for immune checkpoint inhibitor therapy: a systematic review and meta-analysis. Cancer Immunol Immunother 72, 1061–1073 (2023). https://doi.org/10.1007/s00262-022-03328-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03328-9