Abstract
Background
For prognostic evaluation of pancreatic ductal adenocarcinoma (PDAC), the only well-established serum marker is carbohydrate antigen CA19-9. To improve the accuracy of survival prediction, we tested the efficacy of inflammatory serum markers.
Methods
A preoperative serum panel comprising 48 cytokines plus high-sensitivity CRP (hs-CRP) was analyzed in 173 stage I–III PDAC patients. Analysis of the effect of serum markers on survival utilized the Cox regression model, with the most promising cytokines chosen with the aid of the lasso method. We formed a reference model comprising age, gender, tumor stage, adjuvant chemotherapy status, and CA19-9 level. Our prognostic study model incorporated these data plus hs-CRP and the cytokines. We constructed time-dependent ROC curves and calculated an integrated time-averaged area under the curve (iAUC) for both models from 1 to 10 years after surgery.
Results
Hs-CRP and the cytokines CTACK, MIF, IL-1β, IL-3, GRO-α, M-CSF, and SCF, were our choices for the prognostic study model, in which the iAUC was 0.837 (95% CI 0.796–0.902), compared to the reference model’s 0.759 (95% CI 0.691–0.836, NS). These models divided the patients into two groups based on the maximum value of Youden’s index at 7.5 years. In our study model, 60th percentile survival times were 4.5 (95% CI 3.7–NA) years (predicted high-survival group, n = 34) and 1.3 (95% CI 1.0–1.7) years (predicted low-survival group, n = 128), log rank p < 0.001. By the reference model, the 60th percentile survival times were 2.8 (95% CI 2.1–4.4) years (predicted high-survival group, n = 44) and 1.3 (95% CI 1.0–1.7) years (predicted low-survival group, n = 118), log rank p < 0.001.
Conclusion
Hs-CRP and the seven cytokines added to the reference model including CA19-9 are potential prognostic factors for improved survival prediction for PDAC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is an aggressive disease and a worrisome cause of death. In 2018, the estimate was its causing nearly as many deaths (432,000) as cases (459,000) worldwide [1]. Among pancreatic cancer cases, pancreatic ductal adenocarcinoma (PDAC) accounts for over 90%. Curative treatment comprises radical surgery combined with oncological treatment, but only 10–20% of patients are surgically treatable at diagnosis [2, 3]. Even those who do undergo surgery have only a 25% chance of surviving for the next 5 years, with great variance in survival time [4].
The only serum marker for prognostic evaluation prior to surgery is carbohydrate antigen CA19-9, an established PDAC cancer marker. Like CA19-9, C-reactive protein (CRP) has also been associated negatively with survival [5, 6].
Inflammation and immunity display a complex relationship to cancer because they show properties that are both pro-tumor and anti-tumor [7]. Paramount in immune modulation are cytokines. The primary inflammatory cytokines TNF and IL-1 show pro-tumor effects, and IL-1 provokes metastasis [8]. The cytokines may originate from various cell types in the tumor and its microenvironment. Associated with tumor metastasis and recurrence, there has been a shift from TH1 to TH2 cytokines in the gene expression signature. [9]
Hence, the amount of detail that cytokines provide regarding inflammation may prove valuable. We investigated whether a detailed serum cytokine analysis can produce helpful information predicting survival, and whether we can find new potential prognostic factors to improve prediction.
Materials and methods
Patients
Our series comprised 173 consecutive PDAC patients surgically treated at the Department of Surgery, Helsinki University Hospital between 2000 and 2013. Patients with stage IV disease or earlier neoadjuvant therapy were excluded (Fig. 1). Patients with jaundice were endoscopically treated and were non-jaundiced at surgery. The patients presented with no clinical infections or inflammation when samples were gathered.
Cytokine measurements
Preoperative serum samples were stored at − 80 °C and thawed for the first time for the purpose of this study. We analyzed 48 different cytokines by use of the Bio-Plex Pro™ Human Cytokine 27-plex Assay (#M500KCAF0Y) and the 21-plex Assay (#MF0005KMII) (Bio-Rad, Hercules, CA, USA). We had earlier determined the levels of high-sensitivity CRP (hs-CRP) in plasma and CA19-9 in serum [6].
Statistics
We used the Cox regression proportional hazard model to assess the effect of serum values on survival and to determine which variables would best serve for evaluation of patient prognosis. The Benjamini–Hochberg procedure served in calculating adjusted p-values in the univariate analysis (false-discovery rate (FDR) set to 5%, p.adjust function in R). In the multivariate analyses, we also included patient characteristics: age, gender, stage, and adjuvant therapy status. We then fitted lasso (least absolute shrinkage operator) paths for the Cox regression method with tenfold cross-validation to create a penalized Cox regression model and to select potentially important cytokines for the prognostic study model (glmnet package in R) [10, 11]. Bootstrapping (1000 samples) allowed the selection process to determine the overall confidence level for a variable to be included in the model. This was done by calculating the proportion of bootstrapped models in which the individual variable was included in the model. Highly correlated variables (Spearman’s correlation coefficient > 0.8; IL-10, IL-12 p70, and VEGF; IL-17 and MIP-1α; IL-3 and MIG) served as linear combinations in the lasso selection process.
We built a reference prognostic model comprising age, gender, adjuvant therapy status, and serum CA19-9. Our prognostic study model included also hs-CRP and cytokine levels.
We created time-dependent ROC curves for each unpenalized model and integrated the area under the curve (iAUC) from 1 to 10 years (giving an average time-dependent AUC over the time period) to compare the two models (TimeROC package in R) [12]. Bootstrapping (1000 samples) allowed us to determine 95% confidence intervals for the iAUCs. The assumption of the linear risk of a variable was evaluated by construction of restricted cubic splines and tests of the nonlinear component with the Cox regression (rms package in R) [13]. For variables with a suspected nonlinear HR association, if suitable, one cutoff value served to create a binary variable. To demonstrate the models’ function, we used both models to divide our patients into two groups, setting the cutoff point at the maximum value of Youden’s index in the time-dependent ROC curve at 7.5 years.
Results
Among the patients, 10 (6%) had stage IA disease, 15 (9%) stage IB, 32 (18%) stage IIA, 65 (38%) stage IIB and 51 (29%) stage III disease.
Cox univariate analysis gave us significant (p < 0.05) results for (logarithmic) CRP (HR 1.66; 95% CI 1.22–2.27; FDR = 0.025), CA19-9 (HR 1.31; 95% CI 1.11–1.54; FDR = 0.025), and (binary) GRO-α (HR 1.81; 95% CI 1.29–2.54; FDR = 0.025). CA19-9 was also included in the reference model (Table 1). The lasso method provided the seven potential serum variables for the prognostic study model (Table 2): CTACK, MIF, IL-1β, IL-3, GRO-α, M-CSF, and SCF. The latter three were binary variables, whereas the rest were logarithmic. Cutoff points for GRO-α, M-CSF, and SCF were (log) 2,1, 1.1, and 2. CTACK, MIF, IL-3, GRO-α, M-CSF, and SCF had hazard ratios above 1.00, and IL-1β had a hazard ratio below 1.00.
For our prognostic model, the iAUC, describing the average AUC from one to ten years, was 0.837 (95% CI 0.796 –0.902), and for the reference model 0.759 (95% CI 0.691–0.836; nonsignificant difference) (Fig. 2). We also calculated how the reference model would service if combined with hs-CRP; this would result in an iAUC of 0.788 (95% CI 0.728–0.856).
Time-dependent area under the curve for the prognostic model and reference model The integrated area under the curve (iAUC, presenting the time-averaged AUC from one to 10 years) for our prognostic model was 0.837 (95% CI 0.796–0.902) and for the reference model with the iAUC 0.759 (95% CI 0.691–0.836; no significant difference). Dashed lines represent 95% confidence intervals
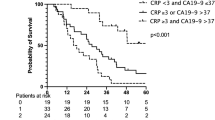
Both the study and the reference model served to divide the patients into two groups, based on the maximum value of Youden’s index on a time-dependent ROC curve at 7.5 years. In our prognostic study model with cytokines and CRP, the 60th percentile survival time was 4.5 (95% CI 3.7–NA) years for the predicted high-survival group and 1.3 (95% CI 1.0–1.7) years for the predicted low-survival group (log rank p < 0.001). The corresponding 60th percentile for survival times was 2.8 (95% CI 2.1–4.4) years (predicted high-survival group) and 1.3 (95% CI 1.0–1.7) years (predicted low-survival group) (log rank p < 0.001), as shown in Fig. 3.
Discussion
We found that adding hs-CRP and inflammatory cytokines CTACK, MIF, IL-1β, IL-3, GRO-α, M-CSF, and SCF to a reference model with serum CA19-9 and patient characteristics improved the survival prediction for PDAC patients. Of these cytokines, IL-1β had a hazard ratio below 1.00, whereas the other cytokines showed hazard ratios above 1.00.
Cancer progression and poor survival are associated with pro-inflammatory cytokines [9]. In pancreatic cancer, evidence exists for changes toward a typical pro-inflammatory cytokine expression: in tissues higher MIF, IL-8, and CXCR-4 expression and reduced HLA-DR expression, and in serum higher IL-6 and IL-10 levels. Similar changes have also been evident in several other cancers. In pancreatic cancer, elevated serum IL-6 and IL-10 concentrations have been linked with worse prognosis, as has the pro-inflammatory cytokine IL-18. In tissue, higher CXCR-4 expression has been associated with metastases and reduced local expression of HLA-DR with worse survival [14].
We found serum MIF, IL-6, IL-8, IL-10, and IL-18 each to have a hazard ratio above 1.00, although only MIF and IL-8 had statistically significant results in either univariate or multivariate analysis. Another study looked at the prognostic effects of the cytokines IL-1β, IL-6, IL-8, IL-10, and TNF-α in PDAC; it showed circulating IL-8 and TNF-α to associate with poor prognosis [15]. Our results concerning the two cytokines were similar, although statistically nonsignificant.
The most widely used marker, CA19-9, has some disadvantages in clinical use. It is of both diagnostic and prognostic value [16,17,18,19,20]. However, those who are Lewis antigen-negative (around 5% of the population) are unable to produce CA19-9 at all [20], leading to possible false diagnoses and incorrect prognoses.
Our prognostic model incorporates pathological cancer stage, i.e., data that are available only postoperatively, but as the cytokines were measured preoperatively, our findings may prove applicable in a preoperative setting as well. With better tools to predict survival, we can treat the patients better.
All patients with jaundice were endoscopically treated before surgery. The patients presented with no infections and inflammatory states before surgery, and we excluded those with the previous neoadjuvant therapy, in order to minimize confounding bias and the risk of externally caused changes in the tumor microenvironment.
The lasso method involves sophisticated statistics incorporating variable selection, regularization, and cross-validation. It helps create the most optimal variable combination for prognostic assessment. We had no external validation set, and hence, our variable selection can be regarded primarily as an investigation to find new potential markers for survival prediction. Nonetheless, we employed bootstrapping to obtain confidence values for each selected variable and to support our findings. Our analysis involved a relatively large number of patients, 173; to our knowledge, no similar studies of this depth have yet appeared.
We hope to guide further research toward the cytokines presented here. It is not entirely understood what exactly causes these cytokines to rise. Presumably it reflects the systemic inflammatory response to the cancer, but it is possible that the elevation is caused by specific processes connected to carcinogenesis or metastasis. Further research is needed to shed light onto this. Ideally, this could lead to new targets for therapeutic cancer treatments. Furthermore, the more accurately a patient’s prognosis can be evaluated preoperatively, the better we know how to choose for them the best available treatment. Our findings may aid in the assessment of PDAC prognosis both pre- and postoperatively, and we call for further studies for validation.
Availability of data and material
The authors confirm that all data supporting the findings of this study are available within the article and in its supplementary material.
Abbreviations
- hs-CRP:
-
High-sensitivity CRP
- iAUC:
-
Integrated time-averaged area under the curve
- lasso:
-
Least absolute shrinkage operator
- PDAC:
-
Pancreatic ductal adenocarcinoma
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Freelove R, Walling AD (2006) Pancreatic cancer: diagnosis and management. Am Fam Physician 73(3):485–492
Hidalgo M (2010) Pancreatic cancer. New Engl J Med 362(17):1605–1617. https://doi.org/10.1056/NEJMra0901557
Seppänen H, Juuti A, Mustonen H, Haapamäki C, Nordling S, Carpelan-Holmström M et al (2017) The results of pancreatic resections and long-term survival for pancreatic ductal adenocarcinoma: a single-institution experience. Scand J Surg 106(1):54–61. https://doi.org/10.1177/1457496916645963
Szkandera J, Stotz M, Absenger G, Stojakovic T, Samonigg H, Kornprat P et al (2014) Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer 110(1):183–188. https://doi.org/10.1038/bjc.2013.701
Salmiheimo A, Mustonen H, Stenman UH, Puolakkainen P, Kemppainen E, Seppänen H et al (2016) Systemic inflammatory response and elevated tumour markers predict worse survival in resectable pancreatic ductal adenocarcinoma. PLoS ONE 11(9):e0163064. https://doi.org/10.1371/journal.pone.0163064
Ostrand-Rosenberg S (2008) Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev 18(1):11–18. https://doi.org/10.1016/j.gde.2007.12.007
Germano G, Allavena P, Mantovani A (2008) Cytokines as a key component of cancer-related inflammation. Cytokine 43(3):374–379. https://doi.org/10.1016/J.CYTO.2008.07.014
Seruga B, Zhang H, Bernstein LJ, Tannock IF (2008) Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 8:887
Simon N, Friedman J, Hastie T, Tibshirani R (2011) Regularization paths for Cox’s proportional hazards model via coordinate descent. J Stat Softw 39(5):1–13
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: http://www.R-project.org/.
Blanche P, Dartigues J, JacqminGadda H (2013) Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Statist Med 32:5381–5397. https://doi.org/10.1002/sim.5958
Department of Biostatics, Vanderbilt university, Nashville, Tennessee. Regression modeling strategies. Available from: http://biostat.mc.vanderbilt.edu/rms
Lippitz BE (2013) Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 14(6):e218–e228. https://doi.org/10.1016/S1470-2045(12)70582-X
Dima SO, Tanase C, Albulescu R, Herlea V, Chivu-Economescu M, Purnichescu-Purtan R et al (2012) An exploratory study of inflammatory cytokines as prognostic biomarkers in patients with ductal pancreatic adenocarcinoma. Pancreas 41(7):1001–1007. https://doi.org/10.1097/MPA.0b013e3182546e13
Goonetilleke KS, Siriwardena AK (2007) Systematic review of carbohydrate antigen (CA 19–9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol 33(3):266–270. https://doi.org/10.1016/j.ejso.2006.10.004
Humphris JL, Chang DK, Johns AL, Scarlett CJ, Pajic M, Jones MD et al (2012) The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol 23(7):1713–22. https://doi.org/10.1093/annonc/mdr561
Berger AC, Garcia M Jr, Hoffman JP, Regine WF, Abrams RA, Safran H et al (2008) Postresection CA 19–9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol 26(36):5918–5922. https://doi.org/10.1200/JCO.2008.18.6288
Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y et al (2005) The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol 31(2):164–169. https://doi.org/10.1016/j.ejso.2004.09.007
Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL (2006) Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol 24(18):2897–2902. https://doi.org/10.1200/JCO.2005.05.3934
Acknowledgements
We thank our research assistants Elina Aspiala and Jaana Koski-Alhainen, and our laboratory technicians Teija Kananen and Maarit Leinimaa.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. This study received financing from the Finnish State Subsidy Fund, the Finnish Cancer Foundation, the Sigrid Jusélius Foundation, the Mary and Georg Ehrnrooth Foundation, Finska Läkaresällskapet, Medicinska Understödsföreningen Liv och Hälsa, and the Finnish Medical Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
This study had the approval of the Helsinki University Hospital Surgical Ethics Committee and the participating surgical departments. It was retrospective in nature, and The Finnish National Supervisory Authority for Welfare and Health gave permission to use this archive material. The cytokine levels were measured without access to patient data. Statistical analysis included complete yet anonymous patient data, including clinic-pathological and survival data.
Consent to participate
Participants had given informed consent to take part in this study.
Consent to publish
There is no identifying information in the article regarding any of the participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lanki, M., Seppänen, H., Mustonen, H. et al. Pancreatic cancer survival prediction via inflammatory serum markers. Cancer Immunol Immunother 71, 2287–2292 (2022). https://doi.org/10.1007/s00262-021-03137-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03137-6