Abstract
Background
Tumor PD-L1 expression is a predictive biomarker for patients with NSCLC receiving PD-(L)1 blockade agents. However, although increased tumor PD-L1 expression predicts responsiveness, clinical benefit has been observed regardless of tumor PD-L1 expression, suggesting the existence of other PD-L1 sources. The aim of our study was to analyze whether integrating systemic and tumor PD-L1 is more predictive of efficacy in patients with advanced NSCLC receiving PD-(L)1 blockade agents.
Material and methods
Twenty-nine healthy donors and 119 consecutive patients with advanced NSCLC treated with PD-(L)1 drug were prospectively included. Pretreatment blood samples were collected to evaluate PD-L1 levels on circulating immune cells, platelets (PLTs), platelet microparticles (PMPs), and the plasma soluble PD-L1 concentration (sPD-L1). Tumor PD-L1 status was assessed by immunohistochemistry. The percentages of circulating PD-L1 + leukocytes, sPD-L1 levels, and tumor PD-L1 were correlated with efficacy.
Results
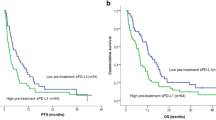
No differences in the percentages of circulating PD-L1 + leukocytes were observed according to tumor PD-L1 expression. Significantly longer progression-free survival was observed in patients with higher percentages of PD-L1 + CD14 + , PD-L1 + neutrophils, PD-L1 + PLTs, and PD-L1 + PMPs and significantly longer overall survival was observed in patients with higher percentages of PD-L1 + CD14 + and high tumor PD-L1 expression. Integrating the PD-L1 data of circulating and tumor PD-L1 results significantly stratified patients according to the efficacy of PD-(L1) blockade agents.
Conclusions
Our results suggest that integrating circulating PD-L1 + leukocytes, PLT, PMPs, and sPD-L1 and tumor PD-L1 expression may be helpful to decide on the best treatment strategy in patients with advanced NSCLC who are candidates for PD-(L)1 blockade agents.






Similar content being viewed by others
Abbreviations
- CH:
-
Chemotherapy
- CI:
-
Confidence interval
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- FITC:
-
Fluorescein
- HD:
-
Healthy donors
- HR:
-
Hazard Ratio
- IO:
-
Immunotherapy
- IPD:
-
Integrated PD-L1 data
- irAEs:
-
Immune-related adverse events
- IHC:
-
Immunohistochemistry
- iRECIST:
-
Immune-Response Evaluation Criteria In Solid Tumors
- NSCLC:
-
Non-small cell lung cancer
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death-ligand 1
- PLTs:
-
Platelets
- PMPs:
-
Platelet microparticles
- sPD-L1:
-
Plasma concentrations of soluble PD-L1
- OS:
-
Overall survival
- PFS:
-
Progression-free-survival
- TPS:
-
Tumor proportion score
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- NE:
-
Not evaluable
- PE:
-
Phycoerythrin
- PECy7:
-
Phycoerythrin Cyanine 7
- PECy5:
-
Phycoerythrin Cyanine 5
References
Nixon NA, Blais N, Ernst S et al (2018) Current landscape of immunotherapy in the treatment of solid tumours, with future opportunities and challenges. Curr Oncol 25:e373–e384. https://doi.org/10.3747/co.25.3840
Bocanegra A, Blanco E, Fernandez-Hinojal G et al (2020) PD-L1 in systemic immunity: unraveling its contribution to PD-1/PD-L1 blockade immunotherapy. Int J Mol Sci 21:1–17
Hui R, Gandhi L, Carcereny Costa E et al (2016) Long-term OS for patients with advanced NSCLC enrolled in the KEYNOTE-001 study of pembrolizumab (pembro). J Clin Oncol 34:9026–9026. https://doi.org/10.1200/jco.2016.34.15_suppl.9026
Hartley G, Regan D, Guth A, Dow S (2017) Regulation of PD-L1 expression on murine tumor-associated monocytes and macrophages by locally produced TNF-α. Cancer Immunol Immunother 66:523–535. https://doi.org/10.1007/s00262-017-1955-5
Hallqvist A, Rohlin A, Raghavan S (2020) Immune checkpoint blockade and biomarkers of clinical response in non–small cell lung cancer. Scand J Immunol 92. https://doi.org/10.1111/sji.12980
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in Cancer. N Engl J Med 366:2443–2454. https://doi.org/10.1056/nejmoa1200690
Cottrell TR, Taube JM (2018) PD-L1 and emerging biomarkers in immune checkpoint blockade therapy. Cancer J (United States) 24:41–46
Prelaj A, Tay R, Ferrara R et al (2019) Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer. Eur J Cancer 106:144–159
Riudavets M, Auclin E, Mezquita L (2020) Host circulating biomarkers for immune-checkpoint inhibitors: single-agent and combinations. Futur Oncol 16:1665–1668
Chen DS, Mellman I (2013) Oncology meets immunology: The cancer-immunity cycle. Immunity 39:1–10
Ilié M, Szafer-Glusman E, Hofman V et al (2018) Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol 29:193–199. https://doi.org/10.1093/annonc/mdx636
Arrieta O, Montes-Servín E, Hernandez-Martinez JM, et al (2017) Expression of PD-1/PD-L1 and PD-L2 in peripheral T-cells from non-small cell lung cancer patients. Oncotarget 8:101994–102005. https://doi.org/10.18632/oncotarget.22025
Bocanegra A, Fernandez-Hinojal G, Zuazo-Ibarra M et al (2019) PD-L1 expression in systemic immune cell populations as a potential predictive biomarker of responses to PD-L1/PD-1 blockade therapy in lung cancer. Int J Mol Sci 20. https://doi.org/10.3390/ijms20071631
Rolfes V, Idel C, Pries R, et al (2018) PD-L1 is expressed on human platelets and is affected by immune checkpoint therapy. Oncotarget 9:27460–27470. https://doi.org/10.18632/oncotarget.25446
Okuma Y, Wakui H, Utsumi H et al (2018) Soluble programmed cell death ligand 1 as a novel biomarker for nivolumab therapy for non–small-cell lung cancer. Clin Lung Cancer 19:410-417.e1. https://doi.org/10.1016/j.cllc.2018.04.014
Zhou J, Mahoney KM, Giobbie-Hurder A et al (2017) Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res 5:480–492. https://doi.org/10.1158/2326-6066.CIR-16-0329
Orme JJ, Jazieh KA, Xie T et al (2020) ADAM10 and ADAM17 cleave PD-L1 to mediate PD-(L)1 inhibitor resistance. Oncoimmunology 9. https://doi.org/10.1080/2162402X.2020.1744980
Chen G, Huang AC, Zhang W et al (2018) (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nat 5607718(560):382–386. https://doi.org/10.1038/s41586-018-0392-8
Orme JJ, Enninga EAL, Lucien-Matteoni F et al (2020) Therapeutic plasma exchange clears circulating soluble PD-L1 and PD-L1-positive extracellular vesicles. J Immunother cancer 8:1–7. https://doi.org/10.1136/jitc-2020-001113
Ando K, Hamada K, Watanabe M, et al (2019) Plasma levels of soluble PD-L1 correlate with tumor regression in patients with lung and gastric cancer treated with immune checkpoint inhibitors. Anticancer Res 39:5195–5201. https://doi.org/10.21873/anticanres.13716
Costantini A, Julie C, Dumenil C et al (2018) Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. Oncoimmunology 7. https://doi.org/10.1080/2162402X.2018.1452581
Ahmad F, Hong HS, Jäckel M et al (2014) High frequencies of polyfunctional CD8+ NK Cells in Chronic HIV-1 infection are associated with slower disease progression. J Virol 88:12397. https://doi.org/10.1128/JVI.01420-14
Lindgren Å, Yun CH, Lundgren A et al (2010) CD8- natural killer cells are greatly enriched in the human gastrointestinal tract and have the capacity to respond to bacteria. J Innate Immun 2:294–302. https://doi.org/10.1159/000286238
Bazhin AV, von Ahn K, Fritz J et al (2018) Interferon-α up-regulates the expression of PD-L1 molecules on immune cells through STAT3 and p38 signaling. Front Immunol 9:2129. https://doi.org/10.3389/fimmu.2018.02129
Munir S, Lundsager MT, Jørgensen MA, et al (2019) Inflammation induced PD-L1-specific T cells. Cell Stress 3:319–327. https://doi.org/10.15698/cst2019.10.201
de Kleijn S, Langereis JD, Leentjens J et al (2013) IFN-γ-stimulated neutrophils suppress lymphocyte proliferation through expression of PD-L1. PLoS ONE 8. https://doi.org/10.1371/journal.pone.0072249
Maine CJ, Aziz NHA, Chatterjee J et al (2014) Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunother 63:215–224. https://doi.org/10.1007/s00262-013-1503-x
Wang WB, Yen ML, Liu KJ et al (2015) Interleukin-25 mediates transcriptional control of PD-L1 via STAT3 in multipotent human mesenchymal stromal cells (hMSCs) to suppress Th17 responses. Stem Cell Reports 5:392–404. https://doi.org/10.1016/j.stemcr.2015.07.013
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255–265. https://doi.org/10.1016/S0140-6736(16)32517-X
Gong L, Cai Y, Zhou X, Yang H (2012) Activated platelets interact with lung cancer cells through P-selectin glycoprotein ligand-1. Pathol Oncol Res 18:989–996. https://doi.org/10.1007/s12253-012-9531-y
Meyo MT, Jouinot A, Giroux-Leprieur E et al (2020) Predictive value of soluble PD-1, PD-L1, VEGFA, CD40 ligand and CD44 for nivolumab therapy in advanced non-small cell lung cancer: a case-control study. Cancers (Basel) 12. https://doi.org/10.3390/cancers12020473
Zhang J, Gao J, Li Y et al (2015) Circulating PD-L1 in NSCLC patients and the correlation between the level of PD-L1 expression and the clinical characteristics. Thorac Cancer 6:534–538. https://doi.org/10.1111/1759-7714.12247
Jacquelot N, Roberti MP, Enot DP et al (2017) Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun 8. https://doi.org/10.1038/s41467-017-00608-2
Brochez L, Meireson A, Chevolet I et al (2018) Challenging PD-L1 expressing cytotoxic T cells as a predictor for response to immunotherapy in melanoma. Nat Commun 9. https://doi.org/10.1038/s41467-018-05047-1
Acknowledgments
SV was supported by “Fondo Investigaciones Sanitarias” and a participant in the Program for the Stabilization of Investigators from the “Direcció i d’Estrategia I Coordinació del Departament Salut de la Generalitat de Catalunya.”
Funding
This work was supported by the Bristol Myers Squibb.
Author information
Authors and Affiliations
Contributions
All authors were involved in revising intellectual content, and all authors approved the final version for publication. CZ, MM, LA, and MAO performed cellular staining and flow cytometry analysis and ELISAs. CZ and MM and MAO analyzed results of flow cytometry and ELISAS; GA, MR, IS, AB, JS, OG, JG, and MM collected samples and clinical data; CZ and SV performed statistical analysis.GA, CZ, MM and SV wrote the manuscript. MM, and SV designed the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was conducted in accordance with the Helsinki Declaration and approved by the Research Ethics Board of Hospital de la Santa Creu I Sant Pau, Barcelona (IIBSP-PDL-2017–82).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zamora Atenza, C., Anguera, G., Riudavets Melià, M. et al. The integration of systemic and tumor PD-L1 as a predictive biomarker of clinical outcomes in patients with advanced NSCLC treated with PD-(L)1blockade agents. Cancer Immunol Immunother 71, 1823–1835 (2022). https://doi.org/10.1007/s00262-021-03107-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03107-y