Abstract
Background
Previous investigations suggest that systemic inflammation markers are able to provide prognostic value in several cancers. This study seeks to characterize the ability of pretreatment platelet-to-lymphocyte ratio (PLR) to prognosticate advanced or metastatic gastric cancer patients (AGC or MGC, respectively) receiving immunotherapy.
Methods
AGC and MGC patients exposed to PD-1 inhibitors from January 2016–August 2021 in the Chinese PLA General Hospital were recruited. Correlations between PLR and overall survival (OS), progression-free survival (PFS), and immunotherapy-associated tumor response rates were determined.
Results
237 patients were enrolled for this retrospective investigation. The 6 month and 12 month PFS based on the area under the curve value was 0.60 and 0.65 (p < 0.05). based on a calculated PLR cut-off value of 139.41, The PLR < 139.41 group has a longer OS in contrast with the PLR ≥ 139.41 group (13.46 m vs 10.71 m, HR = 0.57, 95% CI 0.42–0.78, p = 0.004). The PLR < 139.41 group had a PFS of 7.93 m in contrast to the 4.75 m seen in those with PLR ≥ 139.41 group (HR = 0.57, 95% CI 0.43–0.76, p = 0.002). The disease control rate (DCR) and objective response rate (ORR) were 86.17% and 30.85%, respectively, in the PLR < 139.41 group, but were 82.52% and 32.17%, respectively in the PLR ≥ 139.41 group. Both groups did not show any marked differences in terms of ORR and DCR (p = 0.887, p = 0.476). PLR is an independent prognostic indicator for OS and PFS upon uni- and multivariate analyses (p < 0.05).
Conclusions
Pre-treatment PLR correlated significantly with PFS and OS in AGC and MGC patients who received immunotherapy. An elevated PLR may provide guidance on subsequent treatment options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
As the fifth most frequently encountered malignancy, gastric cancer causes 7.7% of all cancer-associated mortality rate (4th highest) [1]. Immunotherapeutic agents including anti-programmed death protein-1 (PD-1) agents have greatly enhanced the treatment options for advanced or metastatic gastric cancer patients (AGC or MGC, respectively). Although immunotherapy combined with conventional chemotherapy confer improved PFS and OS in AGC as reported in the landmark ATTRICATION4 and Checkmate649 studies [2, 3], a considerable patient population fail to respond to an anti-PD-1 based regimen. Several commonly used indices of systemic inflammation, including the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are known for their prognostic value across various cancers [4,5,6,7,8]. Heightened NLR or PLR predict poor prognosis in gastric cancer individuals who received neoadjuvant or chemotherapy [6, 9,10,11]. A previous study found that NLR levels in AGC patients using third-line nivolumab monotherapy predicted shorter overall survival (OS) [12]. Despite the wealth of knowledge, data is scarce surrounding the relationship between PLR and those treated with anti-pd-1 monotherapy or combined with other agents in AGC or MGC. This study is the first to explore this relationship.
2 Patients and methods
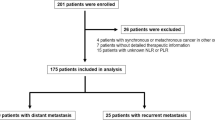
AGC or MGC patients were retrospectively enrolled in this observational experiment. Included individuals were those who received immunotherapy between October 1st 2016 and August 31st 2021 at the Chinese PLA General Hospital. Inclusion criteria were: (1) Histopathological confirmation of gastric cancer; (2) Received two minimum of anti-PD-1 treatment cycles; (3) Presence of measurable lesions; (4) Complete availability of clinicodemographic features such as Eastern Cooperative Oncology Group Performance Status (ECOG PS), serum tumor makers, age, gender and surgery history or nutrition status(based on Nutritional risk screening, NRS-2002), an evaluation of tumor response to treatment; (5) A week-in complete blood count prior to any treatment. The exclusion criteria as follow; (1) Patients treated with either cytotoxic lymphocyte antigen 4 (CTLA4) inhibitors or programmed death protein ligand 1 (PD-L1) antibodies were excluded. (2) Received one cycle of anti-PD-1 treatment and no evaluation outcome. (3) insufficient clinical data for analysis. The Ethical Committee of the Chinese PLA General Hospital provided ethical clearance for this observational retrospective study. Written informed consent was obtained from all patients.
2.1 Treatment and assessment of tumor response
The assortment of treatments received by patients in this study encompassed either monotherapy with PD-1 inhibitors, PD-1 inhibitor + chemotherapy, or anti-angiogenic therapy. PD-1–targeting inhibitors used included nivolumab, pembrolizumab, Sintilimab, or toripalimab. Chemotherapy regimens included the SOX (day 1–14 of twice daily S-1 40–60 mg + day 1 oxaliplatin 130 mg/m2 on day 1) or DCF (cisplatin 75 mg/m2, docetaxel 75 mg/m2 + fluorouracil 750 mg/m2/d) and XELOX (day 1–14 of twice daily capecitabine 1000 mg/m2 for each cycle and + day 1 intravenous oxaliplatin 130 mg/m2 for each cycle) regimens. Anti-angiogenic agents used were either small-molecule tyrosine kinase inhibitors (TKI) (apatinib) or monoclonal antibodies (bevacizumab). Choice of therapy was determined based on patient preference and clinical status.
Tumor degree of response was stratified into either stable disease (SD) or progressive disease (PD), and partial response (PR) or complete response (CR). Disease control was indicated by SD, PR, and CR. CR and PR were also indicative of objective responses. The Evaluation Criteria in Solid Tumors 1.1 assessed tumor response to therapy. Progression-free survival (PFS) was the duration between the time of initiating active therapy until the date of either death or first progression. Overall survival (OS) was the length of time between starting each line of treatment to final follow-up date or death.
2.2 Blood sample analysis
Peripheral blood platelet count and lymphocyte counts taken 1 week-in prior to therapy were recovered from patient records. Division of platelet count with the lymphocyte count yielded the PLR values. Considering the average PFS or OS of late stage gastric cancer is around 6 months or 1 year, the receiver operating characteristic analyses for predicting 6- and 12-month PFS was used to identify an appropriate PLR cutoff value. Patients were then stratified based on this cutoff PLR value.
2.3 Statistical analysis
The SPSS (Windows), version 22 (SPSS Inc., Chicago, IL, USA) was utilized for all data analyses. Median and range were used to describe continuous variables. Categorical data comparisons were done utilizing the Pearson’s chi-square or Fisher’s exact test. The association between PLR and tumor response was assessed using the χ2 test. The Kaplan–Meier method allowed for survival data analysis. Log-rank analysis was used to contrast survival curves. Cox multivariate was analysis was used to verify independent prognostic parameters. The ideal cut-off value was identified using the Youden Index in ROC analysis.
3 Results
The median age of the 237 enrolled patients was 59 years (range, 34–86) and comprised of 109 males and 128 females. The median OS and PFS were 11.41 m and 5.42 m, respectively. 52.74%patients received first-line immunotherapy. Other patients were treated with first- or second-line chemotherapy alone. 181 patients had received immunotherapy combined with chemotherapy, whereas 56 patients received either immunotherapy monotherapy or combined with anti-angiogenic agents. All patients were of the microsatellite stable (MSS) gastric cancer subtype. 112 patients treated with nivolumab and 57 patients with pembrolizumab, 46 patients with Sintilimab, or 22 patients with toripalimab. The median PLR was 199.2(range, 29.9–1375.6). The areas under curve for PLR at 6- and 12-months were 0.60 and 0.65, respectively based on the most prominent point with a sensitivity of 69.7% and specificity of 66.1% (Fig. 1). Based on these values, the optimal cutoff point for PLR at 6-months and 12 months was 139.41 (p < 0.001). Furthermore, it demonstrated significantly different PFS and OS when patients stratified based on PLR quartile values was (p < 0.05) (Fig. 2).
For the ROC analyses, patients were cohorted into PLR < 139.41 group (low PLR) and PLR ≥ 139.41 group (elevated PLR). There was no significant variability in ECOG-PS, gender, age, number of metastatic sites, PD-L1 expression status, smoking and drinking history, primary tumor site, histological differentiation, surgery history, presence of liver metastasis, carbohydrate antigen 19–9 (CA19-9), carcinoembryonic antigen (CEA), and anti-PD-1 type and treatment line or type, risk of malnutrition (Table 1).Of the 237 patients, 30.85% patients in the PLR < 139.41 group demonstrated an objective response (1 CR and 28 PR). This in contrast to only 32.17% in the PLR ≥ 139.41 group. 86.17% of patients in the low PLR group demonstrated confirmed disease control rate (DCR), while only 82.52% achieved this status in the elevated PLR group. The two groups demonstrated no differences in terms of ORR and DCR (p = 0.887, p = 0.476) (Table 2).
Univariate analyses allowed for identification of clinical factors which correlated to PFS or OS. Patients with raised PLR had a median PFS of 4.75 m in contrast to the 7.93 m seen in those with low PLR (HR = 0.57, 95% CI 0.43–0.76, p = 0.002) (Fig. 3). Those with low PLR had a longer median OS than those with elevated PLR (13.46 m vs 10.71 m, HR = 0.57, 95% CI 0.42–0.78, p = 0.004) (Fig. 3). Univariate analysis revealed that those patients with low PLR group or receiving anti-pd-1 plus chemotherapy had better OS than patients with elevated PLR or receiving immunotherapy monotherapy or combined with antigenic agents (P < 0.05) (Table 3).However, multivariate analysis found only PLR to be an independent prognostic biomarker for PFS (HR = 0.58, 95%CI,0.43 -0.78, P < 0.001) (Table 4). PLR and antipd-1 treatment type,surgery history were three independent prognostic factors for OS upon multivariate analysis (HR = 0.59, 95% CI 0.43–0.82, P = 0.001, HR = 1.92, 95% CI 1.29–2.85, P = 0.001; HR = 1.53, 95% CI 1.11–2.11, P = 0.009, respectively) (Table 3). We therefore conclude that elevated PLR was associated with inferior PFS and OS.
4 Discussion
Immunotherapy has been popularized recently in the field of AGC and MGC treatment. Several experiments have researched the ability of routine blood parameters in prognosticating AGC and MGC patients treated with immunotherapy. Parameters such as microsatellite instability, tumor mutation burden (TMB), PD-L1 expression, and tumor infiltrating lymphocyte (TIL) counts have already been identified as markers with likely prognostic value in patients with cancer [13,14,15,16,17]. However, these biomarkers are not commonly available in clinical laboratories, and are time-consuming, require technical expertise, and incur significant cost. Some of these parameters are only available after resection of the primary tumor, which is not feasible in some cases. Therefore, reliable and conveniently available prognostic factors are crucial in guiding patient management.
Systemic inflammation indices such as NLR, PLR, and MLR have previously been affirmed to be able to predict OS in several types of cancers [9, 18,19,20,21,22]. PLR may be a vital player in prognosticating AGC and MGC [11, 23]. However, whether or not PLR holds the same prognostic ability in AGC and MGC patients treated with immunotherapy is not clear. Our study is the first that provides concrete evidence linking PLR to poor OS in this cohort, suggesting that clinicians should be cautious in initiating immunotherapy especially in these patients. Kaplan–Meier survival analysis revealed curves for PFS and OS in patients with pre-treatment PLR < 139.41and PLR ≥ 139.41 that were significantly different. Multivariate analysis further underscored the predictive value of PLR both in PFS and OS. This finding was consistent with other studies in patients treated with chemotherapy [24]. The pathophysiology of this phenomenon has yet to be characterized. We postulate that high PLR correlates to poor OS given that platelet activation features consistently in all steps of tumorigenesis starting from tumor initiation, spread, and metastasis [25]. Additionally, some study showed that platelet also promote tumor metastasis and angiogenesis by releasing various growth factors such as vascular endothelial growth factor-A. The platelet formed can also promote tumor cell immune escape and resistance to chemotherapeutic drugs [24]. Furthermore, lower OS has been observed in cancer patients with thrombocytosis [26]. Decreased T cells on the other hand, is known to indicate improved prognosis across several different cancers. This has been attributed to the ability of these immune regulating cells that hides tumor cells from immune surveillance [27]. Likewise, increased TIL is also linked to increased responsiveness to immune checkpoint therapies [28]. Raised PLR ratios are therefore indicative of a cellular milleu that is highly conducive for tumor growth and poor response to immunotherapy. It is unsurprising that an elevated PLR corresponds to inferior survival due to a high proportion of tumor-promoting platelets and reduction of tumor-killer lymphocytes.
However, our study did not demonstrate a significant ability of PLR for predicting the response rate in AGC or MGC patients treated with PD-1 inhibitors. Similarly, other studies have instead shown a correlation between PLR and chemotherapeutic response [6, 23]. The inconsistencies in results are unclear. It is assumed that chemotherapy exerts toxicity directly on tumor cells while immunotherapy induces a long-term, gradual chemotoxic environment as PFS KM-Curves of keynote177 showed us [29].
Our investigation revealed a calculated cut-off PLR value of 139.41 with an ROC curve based on the 6 or12month PFS. The cut-off PLR level was different compared with previous studies. Jin Wang et al. who also assessed the relationship between PLR and response and survival of patients receiving first-line chemotherapy used a PLR cut-off of 201.6 [23]. The highest PLR cut-off level from a meta-analysis based on 28 studies was at level of 350 [24]. These differences may be attributed to different laboratory reference standards.
Our study is limited by the fact that it is a retrospective study with a small sample size. Furthermore, the values of other inflammatory markers such as NLR and MLR were not calculated. In order for these results to be validated, studies on larger cohorts are necessary. And also, different cutoff value for PLR which might influence the result of study. Lastly, another limitation is variation of treatment regimen and immune checkpoint inhibitors line which can have a significant impact on the outcome of this study.
5 Conclusion
Pre-treatment PLR has prognostic value in AGC and MGC patients treated with immunotherapy. This information is useful in aiding selection of therapy for individuals with gastric cancer.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. https://doi.org/10.3322/caac.21660.
Boku NM, Ryu MH, Oh DY, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/ gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538–37) study. Ann Oncol. 2020. https://doi.org/10.1016/j.annonc.2020.08.2297.
Moehler M, Shitara K, Garrido M, et al. Nivolumab plus chemotherapy versus chemotherapy as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma: first results of the CheckMate 649 study. Ann Oncol. 2020. https://doi.org/10.1200/JCO.2022.40.4_suppl.240.
Xie X, Luo KJ, Hu Y, Wang JY, Chen J. Prognostic value of preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancer. Dis Esophagus. 2016;29(1):79–85.
Miyatani K, Saito H, Kono Y, et al. Combined analysis of the pre- and postoperative neutrophil-lymphocyte ratio predicts the outcomes of patients with gastric cancer. Surg Today. 2018;48(3):300–7.
Hirahara T, Arigami T, Yanagita S, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. 2019;19(1):672.
Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137(2):425–8.
Kim HS, Ku JH. Systemic inflammatory response based on neutrophil-to-lymphocyte ratio as a prognostic marker in bladder cancer. Dis Markers. 2016;2016:8345286.
Kim EY, Lee JW, Yoo HM, Park CH, Song KY. The platelet-to-lymphocyte ratio versus neutrophil-to-lymphocyte ratio: which is better as a prognostic factor in gastric cancer. Ann Surg Oncol. 2015;22(13):4363–70.
Mungan İ, Dicle ÇB, Bektaş Ş, et al. Correction to: does the preoperative platelet-tolymphocyte ratio and neutrophil-tolymphocyte ratio predict morbidity after gastrectomy for gastric cancer. Mil Med Res. 2020;7(1):12.
Ohe Y, Fushida S, Yamaguchi T, et al. Peripheral blood platelet-lymphocyte ratio is good predictor of chemosensitivity and prognosis in gastric cancer patients. Cancer Manag Res. 2020;12:1303–11.
Ota Y, Takahari D, Suzuki T, et al. Changes in the neutrophil-to-lymphocyte ratio during nivolumab monotherapy are associated with gastric cancer survival. Cancer Chemother Pharmacol. 2020;85(2):265–72.
Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–33.
Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65.
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13.
Zhang D, He W, Wu C, et al. Scoring system for tumor-infiltrating lymphocytes and its prognostic value for gastric cancer. Front Immunol. 2019;10:71.
Yagi T, Baba Y, Ishimoto T, et al. PD-L1 expression, tumor-infiltrating lymphocytes, and clinical outcome in patients with surgically resected esophageal cancer. Ann Surg. 2019;269(3):471–8.
Chen L, Zhang F, Sheng XG, Zhang SQ, Chen YT, Liu BW. Peripheral platelet/lymphocyte ratio predicts lymph node metastasis and acts as a superior prognostic factor for cervical cancer when combined with neutrophil: Lymphocyte. Medicine (Baltimore). 2016;95(32): e4381.
Mei Z, Shi L, Wang B, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017;58:1–13.
Zhao QT, Yuan Z, Zhang H, et al. Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: a meta-analysis including 3,720 patients. Int J Cancer. 2016;139(1):164–70.
Wang Z, Peng S, Wang A, et al. Platelet-lymphocyte ratio acts as an independent predictor of prognosis in patients with renal cell carcinoma. Clin Chim Acta. 2018;480:166–72.
Song S, Li C, Li S, Gao H, Lan X, Xue Y. Derived neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio may be better biomarkers for predicting overall survival of patients with advanced gastric cancer. Onco Targets Ther. 2017;10:3145–54.
Wang J, Qu J, Li Z, et al. Pretreatment platelet-to-lymphocyte ratio is associated with the response to first-line chemotherapy and survival in patients with metastatic gastric cancer. J Clin Lab Anal. 2018;32(1): e22185.
Cao W, Yao X, Cen D, Zhi Y, Zhu N, Xu L. The prognostic role of platelet-to-lymphocyte ratio on overall survival in gastric cancer: a systematic review and meta-analysis. BMC Gastroenterol. 2020;20(1):16.
Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11(1):125.
Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. 2018;33(6):965–83.
Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol. 2016;28(8):383–91.
Quigley DA, Kristensen V. Predicting prognosis and therapeutic response from interactions between lymphocytes and tumor cells. Mol Oncol. 2015;9(10):2054–62.
Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: the phase 3 KEYNOTE-177 study. J Clin Oncol. 2020;38(18_suppl):LBA4–LBA4.
Acknowledgements
An earlier version of the manuscript has been presented as a preprint according to the following link "https://www.researchsquare.com/article/rs-690380/v1.
Ethics declarations
Ethical approval and consent to participate
The protocol was approved by The Ethical Committee of the Chinese PLA General Hospital in accordance with the Helsinki Declaration and the national ethical guidelines.
Competing interests
There were no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gou, M., Zhang, Y. Pretreatment platelet-to-lymphocyte ratio (PLR) as a prognosticating indicator for gastric cancer patients receiving immunotherapy. Discov Onc 13, 118 (2022). https://doi.org/10.1007/s12672-022-00571-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-022-00571-5