Abstract
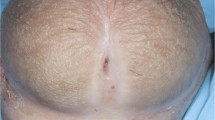
Lichenoid reactions are one of the most frequently observed toxicities with anticancer agents and, recently, a rapid emergence of immunotherapies in oncology has hastened the need to better characterize their unique toxicity profiles, particularly for less common skin toxicities, including anogenital lichen sclerosus et atrophicus (LSA). This case series describes four patients with advanced cancer (one melanoma, two lung cancers, and one kidney tumor) developing LSA lesions while receiving an immunotherapy. Medical records from 2017 to 2020 were retrospectively reviewed. Two patients received pembrolizumab, anti-programmed cell death-1 (PD-1), one nivolumab, anti-programmed cell death-1 (PD-1), and one ipilimumab, an immune checkpoint inhibitor. LSA emerged after a median of 3 months (range, 2–4 months) from starting immunotherapy. All LSA cases were grade 2. Three cases occurred on the penis and one case on the anus. All patients improved after a specific treatment for LSA, and no LSA-related antineoplastic treatment interruption/life-threatening condition were reported. To date, this is the first case series of LSA lesions associated with immunotherapy. Early LSA recognition and management is helpful in cancer patients on immunotherapy allowing a long survival and treatment response.

Similar content being viewed by others
Abbreviations
- IT:
-
Immunotherapy
- LSA:
-
Lichen Sclerosus et Atrophicus
- M:
-
Male
- mo:
-
Months
- N:
-
Number
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- pt:
-
Patient
- yrs:
-
Years
References
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378(2):158–168
Hwang SJ, Carlos G, Wakade D et al (2016) Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol 74(3):455–61.e1
Shi VJ, Rodic N, Gettinger S et al (2016) Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed cell death 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol 152(10):1128–1136
Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW (2019) Lichen Sclerosus: an autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci 15(7):1429–1439
Kreuter A, Kryvosheyeva Y, Terras S et al (2013) Association of autoimmune diseases with lichen sclerosus in 532 male and female patients. Acta Derm Venereol 93:238–241
Hasegawa M, Ishikawa O, Asano Y et al (2018) Diagnostic criteria, severity classification and guidelines of lichen sclerosus et atrophicus. J Dermatol 45(8):891–897
Lewis FM, Tatnall FM, Velangi SS et al (2018) British association of dermatologists guidelines for the management of lichen sclerosus. Br J Dermatol 178(4):839–853
Borghi A, Corazza M, Minghetti S, Bianchini E, Virgili A (2016) Dermoscopic features of vulvar lichen sclerosus in the setting of a prospective cohort of patients: new observations. Dermatology 232(1):71–77
Seyed Jafari SM, Feldmeyer L, Hunger RE (2020) Development of extragenital lichen sclerosus in malignant melanoma patients treated with ipilimumab in combination with nivolumab. Front Oncol. 10:573527
di Meo N, Conforti C, Corneli P et al (2020) Nivolumab-associated extragenital lichen sclerosus et atrophicus. Clin Exp Dermatol 45(3):350–352
Neill SM, Lewis FM, Tatnall FM, Cox NH, Association of Dermatologists B (2010) British Association of Dermatologists’ guidelines for the management of lichen sclerosus 2010. Br J Dermatol 163(4):672–682
Acknowledgements
Patients and their families.
Funding
No funding was required in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Study concept and design: Conteduca, Medri. Acquisition, analysis, or interpretation of data: Conteduca, Medri, Mazzoni, De Giorgi, Stanganelli. Drafting of the manuscript: Conteduca, Medri. Study supervision: Stanganelli. Manuscript review and approval: all authors.
Corresponding author
Ethics declarations
Conflict of interest
V. Conteduca received speaker honoraria or travel support from Astellas, Janssen-Cilag, and Sanofi-Aventis. V. Conteduca received consulting fee from Bayer and is an advisory board member of Janssen, Astellas, AstraZeneca, and Merck. U. De Giorgi reports grants from AstraZeneca; personal fees from Astellas, Bayer, Novartis, Merck Sharp & Dohme, and Pharmamar; grants and personal fees from Sanofi; grants and non-financial support from Roche; and personal fees and non-financial support from Janssen-Cilag, Pfizer, Bristol Myers Squibb, and Ipsen. No potential conflicts of interest were disclosed by the other authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Conteduca, V., Medri, M., Mazzoni, L. et al. Anogenital lichen sclerosus et atrophicus lesions in a case series of cancer patients on immunotherapy. Cancer Immunol Immunother 71, 1545–1548 (2022). https://doi.org/10.1007/s00262-021-03094-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03094-0