Abstract
Introduction
Epstein-Barr virus (EBV) is associated with nasopharyngeal carcinoma (NPC), and provides a target for a dendritic cell (DC) vaccine. CD137 ligand (CD137L) expressed on antigen presenting cells, costimulates CD137-expressing T cells, and reverse CD137L signaling differentiates monocytes to CD137L-DC, a type of DC, which is more potent than classical DC in stimulating T cells.
Methods
In this phase I study, patients with locally recurrent or metastatic NPC were administered CD137L-DC pulsed with EBV antigens (CD137L-DC-EBV-VAX).
Results
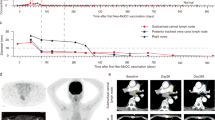
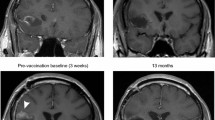
Of the 12 patients treated, 9 received full 7 vaccine doses with a mean administered cell count of 23.9 × 106 per dose. Treatment was well tolerated with only 4 cases of grade 1 related adverse events. A partial response was obtained in 1 patient, and 4 patients are still benefitting from a progression free survival (PFS) of currently 2–3 years. The mean pre-treatment neutrophil: lymphocyte ratio was 3.4 and a value of less than 3 was associated with prolonged median PFS. Progressors were characterized by a high frequency of naïve T cells but a low frequency of CD8+ effector T cells while patients with a clinical benefit (CB) had a high frequency of memory T cells. Patients with CB had lower plasma EBV DNA levels, and a reduction after vaccination.
Conclusion
CD137L-DC-EBV-VAX was well tolerated. The use of CD137L-DC-EBV-VAX is demonstrated to be safe. Consistent results were obtained from all 12 patients, indicating that CD137L-DC-EBV-VAX induces an anti-EBV and anti-NPC immune response, and warranting further studies in patients post effective chemotherapy.
Precis.
The first clinical testing of CD137L-DC, a new type of monocyte-derived DC, finds that CD137L-DC are safe, and that they can induce an immune response against Epstein-Barr virus-associated nasopharyngeal carcinoma that leads to tumor regression or prevents tumor progression.







Similar content being viewed by others
References
Smith C, Khanna R (2012) A new approach for cellular immunotherapy of nasopharyngeal carcinoma. Oncoimmunology 1(8):1440–1442
Tsao SW, Tsang CM, and Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. 2017;372(1732).
Petersson F (2015) Nasopharyngeal carcinoma: a review. Semin Diagn Pathol 32(1):54–73
Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J et al (2016) Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 388(10054):1883–1892
Lv JW, Li JY, Luo LN, Wang ZX, Chen YP (2019) Comparative safety and efficacy of anti-PD-1 monotherapy, chemotherapy alone, and their combination therapy in advanced nasopharyngeal carcinoma: findings from recent advances in landmark trials. J Immunother Cancer 7(1):159
Chow JC, Ngan RK, Cheung KM, Cho WC (2019) Immunotherapeutic approaches in nasopharyngeal carcinoma. Expert Opin Biol Ther 19(11):1165–1172
Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y et al (2014) Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 32(8):798–808
Leung CS, Maurer MA, Meixlsperger S, Lippmann A, Cheong C, Zuo J et al (2013) Robust T-cell stimulation by Epstein-Barr virus-transformed B cells after antigen targeting to DEC-205. Blood 121(9):1584–1594
Sabado RL, Meseck M, Bhardwaj N (2016) Dendritic cell vaccines. Methods Mol Biol 1403:763–777
Gardner A, de Mingo PA, Ruffell B (2020) Dendritic cells and their role in immunotherapy. Front Immunol 11:924
Mastelic-Gavillet B, Balint K, Boudousquie C, Gannon PO, Kandalaft LE (2019) Personalized dendritic cell vaccines-recent breakthroughs and encouraging clinical results. Front Immunol 10:766
Lin CL, Lo WF, Lee TH, Ren Y, Hwang SL, Cheng YF et al (2002) Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Can Res 62(23):6952–6958
Chia WK, Wang WW, Teo M, Tai WM, Lim WT, Tan EH et al (2012) A phase II study evaluating the safety and efficacy of an adenovirus-DeltaLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 23(4):997–1005
Li F, Song D, Lu Y, Zhu H, Chen Z, He X (2013) Delayed-type hypersensitivity (DTH) immune response related with EBV-DNA in nasopharyngeal carcinoma treated with autologous dendritic cell vaccination after radiotherapy. J Immunother 36(3):208–214
Schwarz H, Tuckwell J, Lotz M (1993) A receptor induced by lymphocyte activation (ILA): a new member of the human nerve-growth-factor/tumor-necrosis-factor receptor family. Gene 134(2):295–298
Schwarz H, Valbracht J, Tuckwell J, von KJ, and Lotz M. ILA, the human 4–1BB homologue, is inducible in lymphoid and other cell lineages. Blood. 1995;85(4):1043–52.
Thum E, Shao Z, Schwarz H (2009) CD137, implications in immunity and potential for therapy. Front Biosci 14:4173–4188
Wang S, Chen L (2011) Immunobiology of cancer therapies targeting CD137 and B7–H1/PD-1 cosignal pathways. Curr Top Microbiol Immunol 344:245–267
Vinay DS, Kwon BS (2014) 4–1BB (CD137), an inducible costimulatory receptor, as a specific target for cancer therapy. BMB Rep 47(3):122–129
Guillerey C, Nakamura K, Pichler AC, Barkauskas D, Krumeich S, Stannard K, et al. Chemotherapy followed by anti-CD137 mAb immunotherapy improves disease control in a mouse myeloma model. JCI Insight. 2019;5.
Sanmamed MF, Etxeberria I, Otano I, and Melero I. Twists and turns to translating 4–1BB cancer immunotherapy. Sci Transl Med. 2019;11(496).
Campana D, Schwarz H, Imai C (2014) 4–1BB chimeric antigen receptors. Cancer J 20(2):134–140
Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL et al (2004) Chimeric receptors with 4–1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leuk Off J Leuk Soc Am Leuk Res Fund UK 18(4):676–684
Shao Z, Schwarz H (2011) CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol 89(1):21–29
Pauly S, Broll K, Wittmann M, Giegerich G, Schwarz H (2002) CD137 is expressed by follicular dendritic cells and costimulates B lymphocyte activation in germinal centers. JLeukocBiol 72(1):35–42
Jiang D, Chen Y, Schwarz H (2008) CD137 induces proliferation of murine hematopoietic progenitor cells and differentiation to macrophages. J Immunol 181(6):3923–3932
Jiang D, Yue PS, Drenkard D, Schwarz H (2008) Induction of proliferation and monocytic differentiation of human CD34+ cells by CD137 ligand signaling. Stem Cells 26(9):2372–2381
Jiang D, and Schwarz H. Regulation of granulocyte and macrophage populations of murine bone marrow cells by G-CSF and CD137 protein. PloS one. 2010;5(12):e15565.
Langstein J, Michel J, Fritsche J, Kreutz M, Andreesen R, Schwarz H (1998) CD137 (ILA/4-1BB), a member of the TNF receptor family, induces monocyte activation via bidirectional signaling. J Immunol 160(5):2488–2494
Langstein J, Schwarz H (1999) Identification of CD137 as a potent monocyte survival factor. JLeukocBiol 65(6):829–833
Drenkard D, Becke FM, Langstein J, Spruss T, Kunz-Schughart LA, Tan TE et al (2007) CD137 is expressed on blood vessel walls at sites of inflammation and enhances monocyte migratory activity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 21(2):456–463
Quek BZ, Lim YC, Lin JH, Tan TE, Chan J, Biswas A et al (2010) CD137 enhances monocyte-ICAM-1 interactions in an E-selectin-dependent manner under flow conditions. Mol Immunol 47(9):1839–1847
Zeng Q, Soe YM, Lim Y, Sobota RM, and Schwarz H. CD137 ligand interacts with CD32a to trigger reverse CD137 ligand signaling. Cellular & molecular immunology. 2020.
Sollner L, Shaqireen DOKMM, Wu JT, and Schwarz H. Signal transduction mechanisms of CD137 ligand in human monocytes. Cellular signalling. 2007;19(9):1899–908.
Lippert U, Zachmann K, Ferrari DM, Schwarz H, Brunner E, Latif AH et al (2008) CD137 ligand reverse signaling has multiple functions in human dendritic cells during an adaptive immune response. EurJImmunol 38(4):1024–1032
Kwajah MMS, Schwarz H (2010) CD137 ligand signaling induces human monocyte to dendritic cell differentiation. Eur J Immunol 40(7):1938–1949
Harfuddin Z, Kwajah S, Chong Nyi Sim A, Macary PA, and Schwarz H. CD137L-stimulated dendritic cells are more potent than conventional dendritic cells at eliciting cytotoxic T-cell responses. Oncoimmunology. 2013;2(11):e26859.
Dharmadhikari B, Nickles E, Harfuddin Z, Ishak NDB, Zeng Q, Bertoletti A et al (2018) CD137L dendritic cells induce potent response against cancer-associated viruses and polarize human CD8(+) T cells to Tc1 phenotype. Cancer immunology, immunotherapy : CII 67(6):893–905
Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A et al (2013) Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 38(2):336–348
Harfuddin Z, Dharmadhikari B, Wong SC, Duan K, Poidinger M, Kwajah S et al (2016) Transcriptional and functional characterization of CD137L-dendritic cells identifies a novel dendritic cell phenotype. Sci Rep 6:29712
Zeng Q, Mallilankaraman K, Schwarz H (2019) Increased akt-driven glycolysis is the basis for the higher potency of CD137L-DCs. Front Immunol 10:868
De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN et al (2003) Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Can Res 63(1):12–17
Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK (1999) Migration of human dendritic cells after injection in patients with metastatic malignancies. Can Res 59(1):56–58
Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK et al (2015) Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 519(7543):366–369
Tuyaerts S, Aerts JL, Corthals J, Neyns B, Heirman C, Breckpot K et al (2007) Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer immunology, immunotherapy : CII 56(10):1513–1537
Tan R, Phua SKA, Soong YL, Oon LLE, Chan KS, Lucky SS, et al. Clinical utility of Epstein-Barr virus DNA and other liquid biopsy markers in nasopharyngeal carcinoma. Cancer Commun (Lond). 2020.
Bol KF, Schreibelt G, Gerritsen WR, de Vries IJ, Figdor CG (2016) Dendritic cell-based immunotherapy: state of the art and beyond. Clinical cancer research : an official journal of the American Association for Cancer Research 22(8):1897–1906
Huber A, Dammeijer F, Aerts J, Vroman H (2018) Current state of dendritic cell-based immunotherapy: opportunities for in vitro antigen loading of different DC subsets? Front Immunol 9:2804
Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN (2014) Clinical use of dendritic cells for cancer therapy. Lancet Oncol 15(7):e257–e267
Schuler G (2010) Dendritic cells in cancer immunotherapy. Eur J Immunol 40(8):2123–2130
Mohme M, Neidert MC, Regli L, Weller M, Martin R (2014) Immunological challenges for peptide-based immunotherapy in glioblastoma. Cancer Treat Rev 40(2):248–258
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF et al (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363(5):411–422
Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ (2004) Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Can Res 64(14):4973–4979
Lowenfeld L, Mick R, Datta J, Xu S, Fitzpatrick E, Fisher CS et al (2017) Dendritic cell vaccination enhances immune responses and induces regression of HER2(pos) DCIS independent of route: results of randomized selection design trial. Clinical cancer research : an official journal of the American Association for Cancer Research 23(12):2961–2971
Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P (2019) Neutrophil diversity in health and disease. Trends Immunol 40(7):565–583
Chan KC (2014) Plasma Epstein-Barr virus DNA as a biomarker for nasopharyngeal carcinoma. Chin J Cancer 33(12):598–603
Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P et al (2016) Phase II study of autologous monocyte-derived mrna electroporated dendritic cells (TriMixDC-MEL) Plus Ipilimumab in patients with pretreated advanced melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 34(12):1330–1338
Curran MA, Montalvo W, Yagita H, Allison JP (2010) PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 107(9):4275–4280
Ahlers JD, Belyakov IM (2010) Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood 115(9):1678–1689
Chen S, Lee LF, Fisher TS, Jessen B, Elliott M, Evering W et al (2015) Combination of 4–1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res 3(2):149–160
Woroniecka KI, Rhodin KE, Dechant C, Cui X, Chongsathidkiet P, Wilkinson D et al (2020) 4–1BB Agonism averts TIL exhaustion and licenses PD-1 blockade in Glioblastoma and other intracranial cancers. Clinical cancer research : an official journal of the American Association for Cancer Research 26(6):1349–1358
Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S et al (2016) Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun 7:12624
Chong WQ, Lim CM, Sinha AK, Tan CS, Chan GHJ, Huang Y et al (2020) Integration of Antiangiogenic Therapy with Cisplatin and Gemcitabine Chemotherapy in patients with Nasopharyngeal Carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research 26(20):5320–5328
Lee V, Kwong D, Leung TW, Lam KO, Tong CC, Lee A (2017) Palliative systemic therapy for recurrent or metastatic nasopharyngeal carcinoma - How far have we achieved? Crit Rev Oncol Hematol 114:13–23
Perri F, Della Vittoria Scarpati G, Caponigro F, Ionna F, Longo F, Buonopane S et al (2019) Management of recurrent nasopharyngeal carcinoma: current perspectives. Onco Targets Ther 12:1583–1591
Acknowledgements
We are grateful to the patients who participated. We thank the many people who translated an initial PCR fragment to a clinical application, most notably Joachim Langstein, Daniela Drenkard, Shaqireen Kwajah and Zulkarnain Harfuddin. We thank the Life Sciences Institute flow cytometry core facility for excellent assistance. We thank Lingzhi Wang, Amelia Lau, and Shiwen Qiu for help with analysing EBV plasma DNA levels.
Funding
This research was supported by a grant (NMRC/BnB/018b/2015) from the National Medical Research Council, Singapore.
Author information
Authors and Affiliations
Contributions
Conception and design: Herbert Schwarz, Boon Cher Goh. Financial support: Herbert Schwarz, Boon Cher Goh. Administrative support: Michelle Poon, Yvonne Ang, Yugarajah Asokumaran, Wan Qin Chong, Yiqing Huang, Kwok Seng Loh, Ross Soo. Provision of study materials: Emily Nickles, Bhushan Dharmadhikari, Li Yating, Lip Kun Tan, Mickey Koh, Liam Pock Ho, Marieta Chan, Madelaine Niam, Melissa Soh, Yen Hoon Luah. Provision of patients: Boon Cher Goh, Chwee Ming Lim, J. Walsh, Liang Piu Koh. Collection and assembly of data: Emily Nickles, Li Yating, Robert J. Walsh, Bhushan Dharmadhikari, John E. Connolly, Nivashini Kaliaperumal, Veonice B. Au. Data analysis and interpretation: Herbert Schwarz, Boon Cher Goh, Emily Nickles, Bhushan Dharmadhikari, Li Yating, John E. Connolly, Nivashini Kaliaperumal, Najwa Binte Said Nasir Talib, Reina Sg. Manuscript writing: Herbert Schwarz. Revision and final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Emily Nickles and Bhushan Dharmadhikari authors contributed equally
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nickles, E., Dharmadhikari, B., Yating, L. et al. Dendritic cell therapy with CD137L-DC-EBV-VAX in locally recurrent or metastatic nasopharyngeal carcinoma is safe and confers clinical benefit. Cancer Immunol Immunother 71, 1531–1543 (2022). https://doi.org/10.1007/s00262-021-03075-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03075-3