Abstract
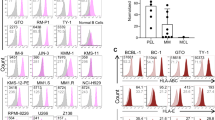
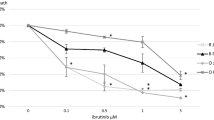
Primary effusion lymphoma (PEL) is a rare, aggressive B cell non-Hodgkin’s lymphoma of the body cavities with malignant effusions. The prognosis is poor, and no optimal treatment has been established. CD38 is a type II transmembrane glycoprotein known to overexpress in multiple myeloma (MM). Daratumumab (DARA), a human CD38-targeting monoclonal antibody (mAb), is approved for MM treatment. In this study, we found expression of CD38 on PEL cells and assessed the anti-PEL activity of DARA. We found that both KHYG-1 and N6 (CD16-transfected KHYG-1) NK cell lines showed direct killing activity against PEL cells with induction of CD107a, and NK-mediated cytotoxicity by N6NK (CD16+) cells increased with DARA treatment. We confirmed direct NK activity and antibody-dependent cell cytotoxicity (ADCC) by expanded NK cells, indicating that DARA has high ADCC activity. We elucidated the antibody-dependent cell phagocytosis (ADCP) by using human monocyte-derived macrophages (MDMs) and mouse peritoneal macrophages. DARA also showed potent complement-dependent cytolysis (CDC) toward PEL. DARA also induced PEL cell death in the presence of a cross-linking antibody. Moreover, treatment with DARA inhibited tumor growth in a PEL xenograft mouse model. These results provide preclinical evidence that Ab targeting of CD38 could be an effective therapeutic strategy for the treatment of PEL.







Similar content being viewed by others
Abbreviations
- ADCC:
-
Antibody-dependent cell cytotoxicity
- ADCP:
-
Antibody-dependent cell phagocytosis
- CDC:
-
Complement-dependent cytolysis
- DARA:
-
Daratumumab
- MM:
-
Multiple myeloma
- MDMs:
-
Human monocyte-derived macrophages
- NHL:
-
Non-Hodgkin’s lymphoma
- PEL:
-
Primary effusion lymphoma
References
Simonelli C, Spina M, Cinelli R, Talamini R, Tedeschi R, Gloghini A, Vaccher E, Carbone A, Tirelli U (2003) Clinical features and outcome of primary effusion lymphoma in HIV-infected patients: a single-institution study. J Clin Oncol 21:3948–3954. https://doi.org/10.1200/JCO.2003.06.013
Chen YB, Rahemtullah A, Hochberg E (2007) Primary effusion lymphoma. Oncologist 12:569–576. https://doi.org/10.1634/theoncologist.12-5-569
Ota Y, Hishima T, Mochizuki M et al (2014) Classification of AIDS-related lymphoma cases between 1987 and 2012 in Japan based on the WHO classification of lymphomas. Cancer Med 3:143–153
Okada S, Goto H, Yotsumoto M (2014) Current status of treatment for primary effusion lymphoma. Intractable Rare Dis Res 3:65–74. https://doi.org/10.5582/irdr.2014.01010
Shimada K, Hayakawa F, Kiyoi H (2018) Biology and management of primary effusion lymphoma. Blood 132:1879–1888. https://doi.org/10.1182/blood-2018-03-791426
Hu Z, Pan Z, Chen W et al (2021) Primary Effusion Lymphoma: A Clinicopathological Study of 70 Cases. Cancers (Basel). https://doi.org/10.3390/cancers13040878
Castillo JJ, Shum H, Lahijani M, Winer ES, Butera JN (2012) Prognosis in primary effusion lymphoma is associated with the number of body cavities involved. Leuk Lymphoma 53:2378–2382. https://doi.org/10.3109/10428194.2012.694075
Boulanger E, Gerard L, Gabarre J, Molina JM, Rapp C, Abino JF, Cadranel J, Chevret S, Oksenhendler E (2005) Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J Clin Oncol 23:4372–4380. https://doi.org/10.1200/JCO.2005.07.084
Coiffier B (2003) Monoclonal antibodies in the management of newly diagnosed, aggressive B-cell lymphoma. Curr Hematol Rep 2:23–29
Czuczman MS, Gregory SA (2010) The future of CD20 monoclonal antibody therapy in B-cell malignancies. Leuk Lymphoma 51:983–994. https://doi.org/10.3109/10428191003717746
Subramanian J, Cavenagh J, Desai B, Jacobs I (2017) Rituximab in the treatment of follicular lymphoma: the future of biosimilars in the evolving therapeutic landscape. Cancer Manag Res 9:131–140. https://doi.org/10.2147/CMAR.S120589
Freeman CL, Sehn LH (2018) A tale of two antibodies: obinutuzumab versus rituximab. Br J Haematol 182:29–45. https://doi.org/10.1111/bjh.15232
Lim ST, Rubin N, Said J, Levine AM (2005) Primary effusion lymphoma: successful treatment with highly active antiretroviral therapy and rituximab. Ann Hematol 84:551–552. https://doi.org/10.1007/s00277-005-1040-6
Bhatt S, Ashlock BM, Natkunam Y, Sujoy V, Chapman JR, Ramos JC, Mesri EA, Lossos IS (2013) CD30 targeting with brentuximab vedotin: a novel therapeutic approach to primary effusion lymphoma. Blood 122:1233–1242. https://doi.org/10.1182/blood-2013-01-481713
de Weers M, Tai YT, van der Veer MS et al (2011) Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186:1840–1848. https://doi.org/10.4049/jimmunol.1003032
Fishwild DM, O’Donnell SL, Bengoechea T et al (1996) High-avidity human IgG kappa monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat Biotechnol 14:845–851. https://doi.org/10.1038/nbt0796-845
Lonberg N, Taylor LD, Harding FA et al (1994) Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature 368:856–859. https://doi.org/10.1038/368856a0
Bhatnagar V, Gormley NJ, Luo L et al (2017) FDA Approval summary: daratumumab for treatment of multiple myeloma after one prior therapy. Oncologist 22:1347–1353. https://doi.org/10.1634/theoncologist.2017-0229
Mikhael J, Richardson P, Usmani SZ et al (2019) A phase 1b study of isatuximab plus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma. Blood 134:123–133. https://doi.org/10.1182/blood-2019-02-895193
Raab MS, Engelhardt M, Blank A et al (2020) MOR202, a novel anti-CD38 monoclonal antibody, in patients with relapsed or refractory multiple myeloma: a first-in-human, multicentre, phase 1–2a trial. Lancet Haematol 7:e381–e394. https://doi.org/10.1016/S2352-3026(19)30249-2
Dhillon S (2020) Isatuximab: first approval. Drugs 80:905–912. https://doi.org/10.1007/s40265-020-01311-1
Liu Q, Kriksunov IA, Graeff R, Munshi C, Lee HC, Hao Q (2005) Crystal structure of human CD38 extracellular domain. Structure 13:1331–1339. https://doi.org/10.1016/j.str.2005.05.012
Lee HC (2006) Structure and enzymatic functions of human CD38. Mol Med 12:317–323. https://doi.org/10.2119/2006-00086.Lee
Malavasi F, Funaro A, Roggero S, Horenstein A, Calosso L, Mehta K (1994) Human CD38: a glycoprotein in search of a function. Immunol Today 15:95–97. https://doi.org/10.1016/0167-5699(94)90148-1
Deaglio S, Mallone R, Baj G et al (2001) Human CD38 and its ligand CD31 define a unique lamina propria T lymphocyte signaling pathway. FASEB J 15:580–582. https://doi.org/10.1096/fj.00-0522fje
Funaro A, Spagnoli GC, Ausiello CM, Alessio M, Roggero S, Delia D, Zaccolo M, Malavasi F (1990) Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. J Immunol 145:2390–2396
Deaglio S, Mallone R, Baj G, Arnulfo A, Surico N, Dianzani U, Mehta K, Malavasi F (2000) CD38/CD31, a receptor/ligand system ruling adhesion and signaling in human leukocytes. Chem Immunol 75:99–120
Deaglio S, Aydin S, Grand MM, Vaisitti T, Bergui L, D’Arena G, Chiorino G, Malavasi F (2010) CD38/CD31 interactions activate genetic pathways leading to proliferation and migration in chronic lymphocytic leukemia cells. Mol Med 16:87–91. https://doi.org/10.2119/molmed.2009.00146
Aarhus R, Graeff RM, Dickey DM, Walseth TF, Lee HC (1995) ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J Biol Chem 270:30327–30333. https://doi.org/10.1074/jbc.270.51.30327
Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, Aydin S (2008) Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 88:841–886. https://doi.org/10.1152/physrev.00035.2007
Billadeau D, Ahmann G, Greipp P, Van Ness B (1993) The bone marrow of multiple myeloma patients contains B cell populations at different stages of differentiation that are clonally related to the malignant plasma cell. J Exp Med 178:1023–1031. https://doi.org/10.1084/jem.178.3.1023
Lin P, Owens R, Tricot G, Wilson CS (2004) Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol 121:482–488. https://doi.org/10.1309/74R4-TB90-BUWH-27JX
Santonocito AM, Consoli U, Bagnato S, Milone G, Palumbo GA, Di Raimondo F, Stagno F, Guglielmo P, Giustolisi R (2004) Flow cytometric detection of aneuploid CD38(++) plasmacells and CD19(+) B-lymphocytes in bone marrow, peripheral blood and PBSC harvest in multiple myeloma patients. Leuk Res 28:469–477. https://doi.org/10.1016/j.leukres.2003.09.015
Koullouros M, Chouari TAM, Stewart A, Kerr K, Dawson D (2017) Isolated cardiac desminopathy. Eur Heart J Cardiovasc Imaging. https://doi.org/10.1093/ehjci/jex049
Mistry JJ, Moore JA, Kumar P et al (2021) Daratumumab inhibits acute myeloid leukaemia metabolic capacity by blocking mitochondrial transfer from mesenchymal stromal cells. Haematologica 106:589–592. https://doi.org/10.3324/haematol.2019.242974
Naik J, Themeli M, de Jong-Korlaar R et al (2019) CD38 as a therapeutic target for adult acute myeloid leukemia and T-cell acute lymphoblastic leukemia. Haematologica 104:e100–e103. https://doi.org/10.3324/haematol.2018.192757
Bride KL, Vincent TL, Im SY et al (2018) Preclinical efficacy of daratumumab in T-cell acute lymphoblastic leukemia. Blood 131:995–999. https://doi.org/10.1182/blood-2017-07-794214
Matas-Cespedes A, Vidal-Crespo A, Rodriguez V et al (2017) The human CD38 monoclonal antibody daratumumab shows antitumor activity and hampers leukemia-microenvironment interactions in chronic lymphocytic leukemia. Clin Cancer Res 23:1493–1505. https://doi.org/10.1158/1078-0432.CCR-15-2095
Goto H, Kojima Y, Nagai H, Okada S (2013) Establishment of a CD4-positive cell line from an AIDS-related primary effusion lymphoma. Int J Hematol 97:624–633. https://doi.org/10.1007/s12185-013-1339-3
Harada H, Saijo K, Watanabe S, Tsuboi K, Nose T, Ishiwata I, Ohno T (2002) Selective expansion of human natural killer cells from peripheral blood mononuclear cells by the cell line. HFWT Jpn J Cancer Res 93:313–319. https://doi.org/10.1111/j.1349-7006.2002.tb02174.x
Hattori S, Matsuda K, Kariya R, Harada H, Okada S (2016) Proliferation of functional human natural killer cells with anti-HIV-1 activity in NOD/SCID/Jak3(null) mice. Microbiol Immunol 60:106–113. https://doi.org/10.1111/1348-0421.12355
Parekh BS, Berger E, Sibley S, Cahya S, Xiao L, LaCerte MA, Vaillancourt P, Wooden S, Gately D (2012) Development and validation of an antibody-dependent cell-mediated cytotoxicity-reporter gene assay. MAbs 4:310–318. https://doi.org/10.4161/mabs.19873
Ghose J, Viola D, Terrazas C et al (2018) Daratumumab induces CD38 internalization and impairs myeloma cell adhesion. Oncoimmunology 7:e1486948. https://doi.org/10.1080/2162402X.2018.1486948
Yamashita M, Kitano S, Aikawa H, Kuchiba A, Hayashi M, Yamamoto N, Tamura K, Hamada A (2016) A novel method for evaluating antibody-dependent cell-mediated cytotoxicity by flowcytometry using cryopreserved human peripheral blood mononuclear cells. Sci Rep 6:19772. https://doi.org/10.1038/srep19772
Baig NA, Taylor RP, Lindorfer MA, Church AK, Laplant BR, Pavey ES, Nowakowski GS, Zent CS (2012) Complement dependent cytotoxicity in chronic lymphocytic leukemia: ofatumumab enhances alemtuzumab complement dependent cytotoxicity and reveals cells resistant to activated complement. Leuk Lymphoma 53:2218–2227. https://doi.org/10.3109/10428194.2012.681657
Goto H, Kojima Y, Matsuda K, Kariya R, Taura M, Kuwahara K, Nagai H, Katano H, Okada S (2014) Efficacy of anti-CD47 antibody-mediated phagocytosis with macrophages against primary effusion lymphoma. Eur J Cancer 50:1836–1846. https://doi.org/10.1016/j.ejca.2014.03.004
Ono A, Hattori S, Kariya R, Iwanaga S, Taura M, Harada H, Suzu S, Okada S (2011) Comparative study of human hematopoietic cell engraftment into BALB/c and C57BL/6 strain of rag-2/jak3 double-deficient mice. J Biomed Biotechnol 2011:539748. https://doi.org/10.1155/2011/539748
Vaeteewoottacharn K, Kariya R, Pothipan P et al (2019) Attenuation of CD47-SIRPalpha signal in cholangiocarcinoma potentiates tumor-associated macrophage-mediated phagocytosis and suppresses intrahepatic metastasis. Transl Oncol 12:217–225. https://doi.org/10.1016/j.tranon.2018.10.007
Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, Leusen JH, Boross P (2016) The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via fcgamma receptor-mediated cross-linking. J Immunol 197:807–813. https://doi.org/10.4049/jimmunol.1501351
Panaampon J, Kudo E, Kariya R, Okada S (2019) Ephedrine enhances HIV-1 reactivation from latency through elevating tumor necrosis factor receptor II (TNFRII) expression. Heliyon 5:e02490. https://doi.org/10.1016/j.heliyon.2019.e02490
Yagita M, Huang CL, Umehara H, Matsuo Y, Tabata R, Miyake M, Konaka Y, Takatsuki K (2000) A novel natural killer cell line (KHYG-1) from a patient with aggressive natural killer cell leukemia carrying a p53 point mutation. Leukemia 14:922–930. https://doi.org/10.1038/sj.leu.2401769
Alpert MD, Heyer LN, Williams DE, Harvey JD, Greenough T, Allhorn M, Evans DT (2012) A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays. J Virol 86:12039–12052. https://doi.org/10.1128/JVI.01650-12
Alter G, Malenfant JM, Altfeld M (2004) CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294:15–22. https://doi.org/10.1016/j.jim.2004.08.008
Clynes RA, Towers TL, Presta LG, Ravetch JV (2000) Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 6:443–446. https://doi.org/10.1038/74704
Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 99:754–758. https://doi.org/10.1182/blood.v99.3.754
Alduaij W, Ivanov A, Honeychurch J et al (2011) Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood 117:4519–4529. https://doi.org/10.1182/blood-2010-07-296913
Cerisano V, Aalto Y, Perdichizzi S et al (2004) Molecular mechanisms of CD99-induced caspase-independent cell death and cell-cell adhesion in Ewing’s sarcoma cells: actin and zyxin as key intracellular mediators. Oncogene 23:5664–5674. https://doi.org/10.1038/sj.onc.1207741
Ivanov A, Beers SA, Walshe CA et al (2009) Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells. J Clin Invest 119:2143–2159. https://doi.org/10.1172/JCI37884
Mateo V, Brown EJ, Biron G, Rubio M, Fischer A, Deist FL, Sarfati M (2002) Mechanisms of CD47-induced caspase-independent cell death in normal and leukemic cells: link between phosphatidylserine exposure and cytoskeleton organization. Blood 100:2882–2890. https://doi.org/10.1182/blood-2001-12-0217
Honeychurch J, Alduaij W, Azizyan M, Cheadle EJ, Pelicano H, Ivanov A, Huang P, Cragg MS, Illidge TM (2012) Antibody-induced nonapoptotic cell death in human lymphoma and leukemia cells is mediated through a novel reactive oxygen species-dependent pathway. Blood 119:3523–3533. https://doi.org/10.1182/blood-2011-12-395541
Velders MP, van Rhijn CM, Oskam E, Fleuren GJ, Warnaar SO, Litvinov SV (1998) The impact of antigen density and antibody affinity on antibody-dependent cellular cytotoxicity: relevance for immunotherapy of carcinomas. Br J Cancer 78:478–483
Lustig HJ, Bianco C (1976) Antibody-mediated cell cytotoxicity in a defined system: regulation by antigen, antibody, and complement. J Immunol 116:253–260
Ferrara C, Stuart F, Sondermann P, Brunker P, Umana P (2006) The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem 281:5032–5036. https://doi.org/10.1074/jbc.M510171200
Shah NN, Singavi AK, Harrington A (2018) Daratumumab in primary effusion lymphoma. N Engl J Med 379:689–690. https://doi.org/10.1056/NEJMc1806295
Cragg MS, Glennie MJ (2004) Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood 103:2738–2743. https://doi.org/10.1182/blood-2003-06-2031
Teeling JL, French RR, Cragg MS et al (2004) Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 104:1793–1800. https://doi.org/10.1182/blood-2004-01-0039
Zent CS, Secreto CR, LaPlant BR, Bone ND, Call TG, Shanafelt TD, Jelinek DF, Tschumper RC, Kay NE (2008) Direct and complement dependent cytotoxicity in CLL cells from patients with high-risk early-intermediate stage chronic lymphocytic leukemia (CLL) treated with alemtuzumab and rituximab. Leuk Res 32:1849–1856. https://doi.org/10.1016/j.leukres.2008.05.014
Dunkelberger JR, Song WC (2010) Complement and its role in innate and adaptive immune responses. Cell Res 20:34–50. https://doi.org/10.1038/cr.2009.139
Kotimaa J, Klar-Mohammad N, Gueler F, Schilders G, Jansen A, Rutjes H, Daha MR, van Kooten C (2016) Sex matters: Systemic complement activity of female C57BL/6J and BALB/cJ mice is limited by serum terminal pathway components. Mol Immunol 76:13–21. https://doi.org/10.1016/j.molimm.2016.06.004
Vidal-Crespo A, Matas-Cespedes A, Rodriguez V et al (2020) Daratumumab displays in vitro and in vivo anti-tumor activity in models of B-cell non-Hodgkin lymphoma and improves responses to standard chemo-immunotherapy regimens. Haematologica 105:1032–1041. https://doi.org/10.3324/haematol.2018.211904
Kurdi AT, Glavey SV, Bezman NA et al (2018) Antibody-dependent cellular phagocytosis by macrophages is a novel mechanism of action of elotuzumab. Mol Cancer Ther 17:1454–1463. https://doi.org/10.1158/1535-7163.MCT-17-0998
Thangaraj JL, Ahn SY, Jung SH et al (2021) Expanded natural killer cells augment the antimyeloma effect of daratumumab, bortezomib, and dexamethasone in a mouse model. Cell Mol Immunol 18:1652–1661. https://doi.org/10.1038/s41423-021-00686-9
Acknowledgements
We are grateful to Ms. Sawako Fujikawa for technical assistance and Ms. Michiko Teramoto for secretarial assistance.
Funding
This work was supported in part by the Research Program on HIV/AIDS (Grant No. 18fk0410008h003) of the Japan Agency for Medical Research and Development and Grants-in-Aid for Science Research (16K08742) from the Ministry of Education, Science, Sports, and Culture of Japan.
Author information
Authors and Affiliations
Contributions
JP contributed to conceptualization, methodology, formal analysis, investigation, and writing—original draft; RK contributed to investigation and methodology; and SO contributed to conceptualization, resources, writing—review and editing, supervision, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Mice were bred and cared for in the animal research facility according to institutional guidelines. All experimental procedures in this study were approved by the Institutional Animal Care and Use Committee at Kumamoto University, Japan.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Panaampon, J., Kariya, R. & Okada, S. Efficacy and mechanism of the anti-CD38 monoclonal antibody Daratumumab against primary effusion lymphoma. Cancer Immunol Immunother 71, 1017–1031 (2022). https://doi.org/10.1007/s00262-021-03054-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03054-8