Abstract
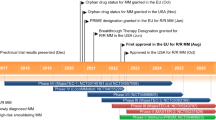
Isatuximab (isatuximab-irfc; Sarclisa®) is an IgG1 monoclonal antibody that binds to the glycoprotein CD38 expressed on the surface of haematopoietic and tumour cells. It is being developed by Sanofi, under a license from Immunogen, for the treatment of haematological malignancies. In March 2020, intravenous isatuximab (in combination with pomalidomide and dexamethasone) was approved in the USA for the treatment of adult patients with multiple myeloma who have received ≥ 2 prior therapies, including lenalidomide and a proteasome inhibitor. Isatuximab has also received a positive opinion in the EU for the treatment of relapsed and refractory multiple myeloma. This article summarizes the milestones in the development of isatuximab leading to the first approval in the USA.
Similar content being viewed by others
Change history
23 May 2020
In the table ‘Key clinical trials of isatuximab (Sanofi)’, in the left-hand column.
References
van de Donk N, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131(1):13–29.
van de Donk NW, Janmaat ML, Mutis T, et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270(1):95–112.
US Food & Drug Administration. FDA approves isatuximab-irfc for multiple myeloma [media release]. 2020. https://www.fda.gov/drugs/development-approval-process-drugs/fda-approves-isatuximab-irfc-multiple-myeloma.
Sanofi-aventis. SARCLISA® (isatuximab-irfc): US Prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761113s000lbl.pdf. Accessed 4 Mar 2020.
Sanofi. Sanofi receives positive CHMP opinion for Sarclisa® (isatuximab) for the treatment of relapsed and refractory multiple myeloma [media release]. 2020. https://www.sanofi.com/-/media/Project/One-Sanofi-Web/Websites/Global/Sanofi-COM/Home/media-room/press-releases/2020/2020-03-27-07-05-00-2007411-en.pdf.
ImmunoGen. ImmunoGen and Sanofi amend license agreements [media release]. 2017. http://investor.immunogen.com/news-releases/news-release-details/immunogen-and-sanofi-amend-license-agreements.
Sanofi. Sanofi delivers 2017 business EPS in line with guidance [media release]. 2018. http://www.news.sanofi.us/2018-02-07-Sanofi-Delivers-2017-Business-EPS-in-line-with-Guidance.
Deckert J, Wetzel MC, Bartle LM, et al. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res. 2014;20(17):4574–83.
Martin TG, Corzo K, Chiron M, et al. Therapeutic opportunities with pharmacological inhibition of CD38 with isatuximab. Cells. 2019;8:12.
Feng X, Zhang L, Acharya C, et al. Targeting CD38 suppresses induction and function of T regulatory cells to mitigate immunosuppression in multiple myeloma. Clin Cancer Res. 2017;23(15):4290–300.
Moreno L, Perez C, Zabaleta A, et al. The mechanism of action of the anti-CD38 monoclonal antibody isatuximab in multiple myeloma. Clin Cancer Res. 2019;25(10):3176–87.
Jiang H, Acharya C, An G, et al. SAR650984 directly induces multiple myeloma cell death via lysosomal-associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia. 2016;30(2):399–408.
Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096–107.
Richardson PG, Attal M, Campana F, et al. Isatuximab plus pomalidomide/dexamethasone versus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma: ICARIA Phase III study design. Future Oncol. 2018;14(11):1035–47.
Harrison SJ, Richardson PG, Alegre A, et al. Efficacy of isatuximab/pomalidomide/dexamethasone in relapsed/refractory multiple myeloma: ICARIA-MM high-risk cytogenetics subgroup analysis [abstract no. OAB-048]. Clin Lymphoma Myeloma Leukemia. 2019;19(10 Suppl):e33.
Schjesvold FH, Richardson PG, Attal M, et al. Efficacy of isatuximab with pomalidomide and dexamethasone in elderly patients with relapsed/refractory multiple myeloma: ICARIA-MM subgroup analysis [abstract no. 1893 plus poster]. Blood. 2019;134(Suppl):1.
Dimopoulos MA, Leleu X, Moreau P, et al. Effect of isatuximab plus pomalidomide/dexamethasone on renal impairment in relapsed/refractory multiple myeloma: ICARIA-MM study subgroup analysis [abstract no. SP-094]. Clin Lymphoma Myeloma Leukemia. 2019;19(10 Suppl):e254.
Ikeda T, Sunami K, Huang SY, et al. Efficacy and safety of isatuximab plus pomalidomide and dexamethasone in East Asian patients with relapsed/refractory multiple myeloma: a subgroup analysis of ICARIA-MM study [abstract no. 2690]. Ann Oncol. 2019;30(Suppl 9):ix92.
Hulin C, Richardson PG, Attal M, et al. Depth of response and response kinetics in the ICARIA-MM study of isatuximab with pomalidomide/dexamethasone in relapsed/refractory multiple myeloma [abstract no. 3185]. Blood. 2019;134(Suppl):1.
Houghton K, Dimopoulos MA, Lin P, et al. Health-related quality of life in patients with relapsed/refractory multiple myeloma treated with isatuximab plus pomalidomide and dexamethasone: Icaria-mm study [abstract no. 1850]. Blood. 2019;134(Suppl):1.
Martin T, Strickland S, Glenn M, et al. Phase I trial of isatuximab monotherapy in the treatment of refractory multiple myeloma. Blood Cancer J. 2019;9(4):41.
Dimopoulos M, Bringhen S, Anttila P, et al. Results from a phase II study of isatuximab as a single agent and in combination with dexamethasone in patients with relapsed/refractory multiple myeloma [abstract no. 155 and oral presentation]. Blood. 2018;132(Suppl):1.
Richter JR, Martin TG, Vij R, et al. Updated data from a phase II dose finding trial of single agent isatuximab (SAR650984, anti-CD38 mAb) in relapsed/refractory multiple myeloma (RRMM) [abstract no. 8005]. J Clin Oncol. 2016;34:15.
Iida S, Sunami K, Ri M, et al. Phase 1/2 Study of isatuximab monotherapy for relapsed and/or refractory multiple myeloma in Japanese patients [abstract no. PF623]. HemaSphere. 2019;3(Suppl 1):265.
Martin T, Baz R, Benson DM, et al. A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017;129(25):3294–303.
Richter J, Wong S, Chari A, et al. Phase I-b study of isatuximab + carfilzomib in relapsed and refractory multiple myeloma (RRMM) [abstract no. PF582]. HemaSphere. 2018;2(Suppl 2):241–2.
Mikhael J, Richardson P, Usmani SZ, et al. A phase 1b study of isatuximab plus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma. Blood. 2019;134(2):123–33.
Usmani S, Karanes C, Bensinger W, et al. Preliminary data: phase 1B study of feasibility/safety of isatuximab short duration fixed volume infusion in combination with pomalidomide and dexamethasone for relapsed/refractory multiple myeloma [abstract no. PS1390]. HemaSphere. 2019;39(Suppl 1):637–8.
Luetkens T, Yousef S, Shorter C, et al. “In vivo vaccination” effect in clinical responders to anti-myeloma monoclonal antibody isatuximab [abstract no. 1830]. Blood. 2017;130(Suppl):1.
Atanackovic D, Yousef S, Shorter C, et al. In vivo vaccination effect in multiple myeloma patients treated with the monoclonal antibody isatuximab. Leukemia. 2020;34(1):317–21.
Asemissen AM, Autenrieth S, Bieber K, et al. Prospective monitoring of immune signatures in newly diagnosed high risk multiple myeloma patients under treatment with isatuximab, carfilzomib, lenalidomide and dexamethasone (I-KRd): first results of the GMMG-CONCEPT trial [abstract no. FP-151]. Clin Lymphoma Myeloma Leukemia. 2019;19(10 Suppl):e141–2.
Weisel K, Asemissen AM, Schieferdecker A, et al. Isatuximab, carfilzomib, lenalidomide and dexamethasone (I-KRd) in front-line treatment of high-risk multiple myeloma: results of the safety run-in cohort in the phase II, multicenter GMMG-CONCEPT trial [abstract no. OAB-023]. Clin Lymphoma Myeloma and Leukemia. 2019;19(10 Suppl):e17.
Ocio EM, Bringhen S, Oliva S, et al. A phase Ib study of isatuximab in combination with bortezomib, cyclophosphamide, and dexamethasone (VCDI) in patients with newly diagnosed multiple myeloma non-eligible for transplantation [abstract no. 3160]. Blood. 2017;130(Suppl):1.
Manasanch EE, Jagannath S, Lee HC, et al. A multicenter phase II single arm trial of isatuximab in patients with high risk smoldering multiple myeloma (HRSMM) [abstract no. 3116]. Blood. 2019;134(Suppl):1.
Moreau P, Dimopoulos MA, Yong K, et al. Isatuximab plus carfilzomib/dexamethasone versus carfilzomib/dexamethasone in patients with relapsed/refractory multiple myeloma: IKEMA phase III study design. Future Oncol. 2020;16(2):4347–58.
Facon T, Goldschmidt H, Cavo M, et al. Phase III (IMROZ) study design: Isatuximab plus bortezomib, lenalidomide, and dexamethasone (VRD) versus VRD in transplant-ineligible patients with newly diagnosed multiple myeloma [abstract no. PB2185]. HemaSphere. 2018;2(Suppl 2):976.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. S. Dhillon is a contracted employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The original article has been updated: due to Page 6 Table update.
Enhanced material for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.12101598.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Dhillon, S. Isatuximab: First Approval. Drugs 80, 905–912 (2020). https://doi.org/10.1007/s40265-020-01311-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01311-1