Abstract
Objectives
A minority of NSCLC patients benefit from anti-PD1 immune checkpoint inhibitors. A rational combination of biomarkers is needed. The objective was to determine the predictive value of tumor mutational load (TML), CD8+ T cell infiltration, HLA class-I and PD-L1 expression in the tumor.
Materials and methods
Metastatic NSCLC patients were prospectively included in an immune-monitoring trial (NTR7015) between April 2016-August 2017, retrospectively analyzed in FFPE tissue for TML (NGS: 409 cancer-related-genes) and by IHC staining to score PD-L1, CD8+ T cell infiltration, HLA class-I. PFS (RECISTv1.1) and OS were analyzed by Kaplan–Meier methodology.
Results
30 patients with adenocarcinoma (67%) or squamous cell carcinoma (33%) were included. High TML was associated with better PFS (p = 0.004) and OS (p = 0.025). Interaction analyses revealed that patients with both high TML and high total CD8+ T cell infiltrate (p = 0.023) or no loss of HLA class-I (p = 0.026), patients with high total CD8+ T cell infiltrate and no loss of HLA class-I (p = 0.041) or patients with both high PD-L1 and high TML (p = 0.003) or no loss of HLA class-I (p = 0.032) were significantly associated with better PFS. Unsupervised cluster analysis based on these markers revealed three sub-clusters, of which cluster-1A was overrepresented by patients with progressive disease (15 out of 16), with significant effect on PFS (p = 0.007).
Conclusion
This proof-of-concept study suggests that a combination of PD-L1 expression, TML, CD8+ T cell infiltration and HLA class-I functions as a better predictive biomarker for response to anti-PD-1 immunotherapy. Consequently, refinement of this set of biomarkers and validation in a larger set of patients is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumors evade T-cell mediated destruction by exploiting inhibitory immune checkpoints such as the PD-1/ PD-L1 pathway. The efficacy of treatment with immune checkpoint inhibitors (ICIs) targeting this pathway in non–small-cell lung cancer (NSCLC) is limited, and better use of biomarkers is needed to predict response to treatment [3].
The currently most widely used biomarker is PD-L1 expression in the tumor, as assessed by the PD-L1 tumor proportion score (TPS), which is positively associated with a response to ICI treatment in metastatic NSCLC patients [4]. However, the performance of the PD-L1 assay to predict clinical response remains poor [5].
The presence of tumor-infiltrating CD8+ T cells which recognize tumor antigens, when presented at the tumor cell surface in the context of HLA class I, is a prerequisite for successful ICI treatment. A surrogate marker for recognition of tumor antigens is tumor mutational load (TML), a measurement of the frequency of mutations in tumor cells, that correlates with the number of neoantigens that can be recognized by CD8+ T cells [6]. Next-generation genome sequencing (NGS) panels composed of about 300–600 cancer-related genes are designed to predict the TML with similar accuracy as whole-exome sequencing [7]. A strong CD8+ type 1 T cell infiltration of tumors critically contributes to a better clinical outcome in cancer, including NSCLC [6]. Conversely, (partial) loss of HLA occurs in a sizeable fraction of NSCLC tumors, as well as HLA diversity modulate the prognostic impact of tumor-infiltrating CD8+ T cells, and thus survival after checkpoint blockade [8,9,10,11]. While TML is an emerging biomarker, CD8+ T cell infiltration and HLA expression have not been considered as predictive biomarkers in NSCLC.
Therefore, this study is the first to determine the predictive value of the TML, CD8+ T cells and HLA class I expression in combination with the PD-L1 expression in anti-PD-1 treated NSCLC.
Materials and methods
Study population
Patients with stage IV NSCLC who started nivolumab monotherapy between April 2016 and August 2017 at the Erasmus University Medical Center, Rotterdam, The Netherlands, were included prospectively in the MULTOMAB study (Dutch Trial Registry NTR7015/NL6828). The study was approved by the independent ethics committee (Medical Research Ethics Committee Erasmus MC; MEC 16-011) and all patients provided written informed consent. Patients were randomly selected and assessed for eligibility. Patients with NSCLC stage IV were included who had been treated with nivolumab monotherapy (weight-based dosing: 3 mg/kg i.v., Q2W) and who were evaluable by RECIST v1.1. Patients who were treated with a prior line of immunotherapy were excluded. The median follow-up time was 27 weeks (interquartile range 14–46 weeks) and the median time between the diagnostic biopsy and first administration of nivolumab was 5 weeks (interquartile range 1–41 weeks). The use of archival formalin-fixed, paraffin-embedded (FFPE) samples was in accordance with guidelines from the Dutch Federation of Medical Research and was approved by the independent ethics committee (Medical Research Ethics Committee Erasmus MC; MEC 17-1186). Specimen handling and all biomarker assay analyses were undertaken blinded; a unique code was assigned for each patient, with a separate list linking these codes with the patient characteristics and outcomes.
TML assay
Mutational load was determined by the Oncomine TML assay (ThermoFisher Scientific, Waltham, MA) according to manufacturer’s protocol on an Ion Torrent S5 XL next-generation sequencing platform (Gilford, NH). Mutational load is defined as the number of somatic nonsynonymous variants (missense and nonsense single nucleotide variants plus insertions and deletions) detected per megabase of exonic sequence with sufficient coverage. Germline variants were filtered out using the Mutation Load Calculation Filter Chain in Ion Reporter software 5.10 (ThermoFisher Scientific, Waltham, MA). A TML cut-off of 11 mut/Mb was used to differentiate between tumors with low or high TML.
Immunohistochemistry on FFPE samples
Expression patterns of classical HLA (HLA-A and HLA-B/C) were assessed according to the Ruiter scoring system [12] as described before [10]. The intensity and percentage of cells in the tumor were determined based on the sum of the intensity of staining (ranging from 0–3) and percentage positive cells (ranging from 0–5). Loss of HLA class I was defined by a low Ruiter score (0–3) of both HLA-A and HLA-B/C. Patients were dichotomized for low or high total CD8+ T cell infiltration based on the mean CD8+ T cell infiltration for all patients and for low (< 50%) or high (≥ 50%) PD-L1 TPS (using the ready-to-use SP263 Ab clone on a Ventana Benchmark Ultra (both form Roche Diagnostics, Tucson, AZ) system according to the manufacturer’s instructions. Mouse monoclonal Abs HCA-2 and HC-10 (tissue culture supernatant respectively anti HLA-A, 1:500, and anti HLA-B/-C, 1:750; a generous gift from Prof. dr. J. Neefjes, Department of Cell and Chemical Biology, LUMC) were used to detect the free heavy chain of the classical HLA-A and HLA- B/-C molecule). The detection of CD8+ T-cells was done using mouse monoclonal CD8 Ab (clone IA5, Leica Biosystems, Germany, 1:500). PD-L1 TPS was determined using clone SP263 (Ventana PD-L1 assay, Roche, Switzerland).
Statistical analysis
Best overall response (BOR) was assessed according to RECIST v1.1 for complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD): minimum duration of 90 days for SD was required, confirmation of CR or PR was not necessary. PFS was defined as the time between the first administration of nivolumab until PD or death due to any cause, and OS until death due to any case. Survival was compared by log-rank test using Kaplan–Meier methodology. Group comparisons of categorical data were performed by 2-tailed χ2 or Fisher’s Exact test. Differences with two-sided P values < 0.05 were considered significant. No power analysis was performed in this proof-of-concept study. R version 1.1.453 (R-project, www.rproject.org) was used for hierarchical cluster analysis with complete linkage by Manhattan distance measure, using the mean for missing values, statistical software package SPSS v24.0.0.1 (SPSS, Chicago, USA) was used for further statistical analysis.
Results
A total of 99 patients were assessed for eligibility, of whom 69 patients were excluded because of either insufficient or poor quality of samples (n = 33), or failure to obtain FFPE material from referring hospitals (n = 36). 30 patients were analyzed (Table 1). The mean duration of nivolumab treatment was 5.4 months (SD: 4.6). Two patients (6%) developed severe immune-related toxicity (grade 3/4, according to CTCAE 4.03). All patients had at least one prior line of chemotherapy, consisting of platinum-based doublet chemotherapy, and three patients were also treated with an EGFR tyrosine kinase inhibitor. The mean duration of response to first-line chemotherapy was 7.2 months (SD: 4.7). Examples of two representative patients are shown in Fig. 1a, displaying TML and IHC staining of classical HLA, CD8+ T cells and PD-L1.
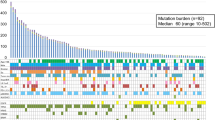
Patient examples and cluster analysis. a Example of two patients showing the BOR (RECIST v1.1): TML NGS output, and the IHC of HLA-A, HLA-B/C, total CD8+ and PD-L1. For HLA, the percentage of positive tumor cells was classified (0–5) and the intensity of the staining (0–3), resulting in a final score based on both (0–8) and was categorized as 0–3 (low) or 4–8 (high); according to the Ruiter scoring system. Loss of classical HLA was defined as absent expression (0–3) of both HLA-A and HLA-B/C IHC. Magnification × 20. b Heat map of unsupervised cluster analysis based on classical HLA, total CD8 tumor infiltration, TML and PD-L1 revealing three distinct clusters (1–3). BOR by RECIST v1.1 was incorporated in the heat map
First, the prognostic effect of each parameter on PFS (Fig. 2a–d) and OS (Fig. S1a-d) was determined. High TML was significantly associated with better PFS (p = 0.004) and OS (p = 0.025). PD-L1 was associated with improved PFS (p = 0.027), but not with OS (p = 0.121). CD8+ T cells and HLA as individual biomarkers were not significantly associated with better OS or PFS, which was expected [10], although normal expression of HLA class I resulted in the better OS and PFS when compared to complete or partial loss of HLA expression. Complete loss was defined by a low score (0–2), partial loss by an intermediate score (3–6), and normal expression by a high score (7–8). Patients with complete loss had impaired PFS compared to patients with partial loss or normal expression of HLA class I (Fig. S2).
PFS analysis. Kaplan–Meier plots showing the PFS by a TML high (> 11 mut/Mb) vs. low (< 11 mut/Mb), b CD8+ T cell infiltration high vs. low, c PD-L1 high (> 50%) vs low, d classical HLA (-A and –B/C) loss vs. rest, e TML high and total CD8+ high vs. rest, f TML high and no loss of classical HLA vs. rest, g total CD8+ high and no loss of classical HLA vs. rest, h TML high and PD-L1 high vs. rest, i PD-L1 high and no loss of classical HLA vs. rest, j PD-L1 high and CD8+ high vs. rest and k cluster (cluster 1A, 1B and 2)
However, interaction analyses between these two markers and the other markers revealed that each combination of two markers was significantly associated with better PFS (Fig. 2e–j), except for PD-L1 with CD8+ T cells due to low power. Specifically, (1) high TML and either high total CD8+ T cell infiltration (p = 0.023) or no loss of HLA class I (p = 0.026), (2) high total CD8+ T cell infiltration and no loss of HLA class I (p = 0.041), and (3) high PD-L1 and either high TML (p = 0.003) or no loss of HLA class I (p = 0.032) was associated with better PFS.
Next, the patients were divided into two groups on the basis of a clinical response (CR/PR/SD) or failure to respond (PD) to nivolumab treatment (Table S1). This revealed a significant overrepresentation of patients with a high TML (p = 0.043) and/or more profound total CD8+ T cell infiltration (p = 0.005) among clinical responders. This association was not found for HLA class I expression or PD-L1 (TPS ≥ 1% or ≥ 50%).
A comparison of the absolute values for all these parameters confirmed that the mean TML (p = 0.001) and mean total CD8+ T cell infiltrate (p = 0.004) were higher in the group of patients with a treatment response (Table S2). Notably, the TML was not directly correlated with CD8+ T-cell infiltration or HLA expression but was positively associated with PD-L1 (p = 0.035; Table S3).
Finally, an unsupervised cluster analysis based on the four parameters was performed. This revealed two major clusters and a total of three sub-clusters (Fig. 1b). Cluster 1A was overrepresented by patients with PD (15 out of 16) of which the tumors were negative for 2–3 of the 4 biomarkers. Cluster 2 almost exclusively comprised patients with SD of which the tumor was positive for 2–3 biomarkers but did not express PD-L1. Survival analyses of the 3 different clusters (Figs. 2k, S1k) indicate a significant effect on PFS (p = 0.007), while the OS (p = 0.74) was significant in a post-hoc comparison of cluster 1A with 1B (p = 0.048).
Discussion
The present proof-of-concept study suggests that in addition to PD-L1 expression also TML, CD8+ T cell infiltration and HLA class I expression are associated with PFS and predict the response to anti-PD-1 immunotherapy. Interestingly, unsupervised cluster analysis of the patients based on all four markers revealed one cluster pattern that almost exclusively identified non-responders (cluster 1A). In the current real-life setting, only a small amount of archival material could be used for TML determination and IHC staining, derived from routine biopsy specimens from the primary tumor and following initial diagnostic procedures (including routine NGS testing for driver mutations in some cases). We were able to demonstrate the clinical value of TML analysis in this immuno-oncology setting, and we believe this is the first study to do so in combination with a range of IHC biomarkers in small, realistic biopsy specimens.
Our findings are consistent with previous studies in different settings, although those studies cannot be generalized. Associations between TMB and immune signatures are generally cancer type dependent [13], and it can be assumed that they are also tumor stage dependent. In addition, a prospective study in early-stage untreated NSCLC patients demonstrated a significant and independent association of low immune-evasion capacity (defined as tumors with no immune editing potential, no HLA loss and no antigen processing machinery [APM] defects) and high number of neoantigens with increased disease-free survival [14].
Moreover, the release of checkpoint blockade by nivolumab may result in a series of dynamic changes in the composition of the tumor microenvironment [15] which override the current prediction (false negatives), but this was not taken into account as we were limited to the use of archival material prior to ICI therapy. Notably, a significant correlation between PD-L1 expression and TML was determined, which may contradict accumulating evidence from clinical trials [16]. This may be related to the limited number of patients, but may also result from the use of continuous covariates rather than stratified data in clinical trials where true correlations may easily be overlooked. Based on our findings, it could not be established that TML and PD-L1 serve as an independent biomarker for clinical outcome.
An interesting finding of this study is the added value of expression of HLA class I molecules on cancer cells, which is known to be crucial for the recognition of tumor cells by CD8 + TCs. In our opinion, the actual detection of HLA class I expression is more valuable as a future biomarker for ICIs than genetic, epigenetic, transcriptional, post-transcriptional or post-translational aberrations, such as loss of heterozygosity in HLA or B2M mutations, since protein expression is the ultimate outcome of all those changes. For instance, genetic studies have revealed that NLRC5, an HLA class I transactivator, is an important target for cancer immune evasion. The expression of NLRC5 correlated with that of HLA class I and negatively correlated with OS in stage III NSCLC [17]. We focused primarily on the expression of HLA class I and did not determine selective APM defects, while this could also affect the recognition of tumor antigens by the immune system. For HLA peptides to be presented to CD8 + T cells, peptides must be processed by proteolysis, trimmed by enzymes to fit into the groove of HLA molecules, and transported intracellularly by peptide transporters, endoplasmic reticulum chaperones and the Golgi apparatus. The antigen presenting pathway often is altered in cancer, including lung cancer [18, 19]. Further studies should be directed at investigating the impact of the APM defects on response to ICIs. Last but not least, due to the relatively low patient numbers, we decided to take complete loss defined as a low score (0–3) of both HLA-A and HLA-B/C, but not partial HLA class I loss into consideration. However, a subgroup analysis showed that patients with complete loss have impaired OS and PFS compared to patients with no loss of HLA class I. The PFS of patients with partial loss was comparable to that of patients with no loss of HLA class I.
Taken together, the findings support the hypothesis that a rational combination of biomarkers—based on the biological requirements for the ICIs to work—may contribute to a more adequate response prediction of ICI treatment in NSCLC. Consequently, the refinement of this proposed set of biomarkers and validation in a greater set of patients is warranted.
Abbreviations
- APM:
-
Antigen processing machinery
- CR:
-
Complete response
- FFPE:
-
Formalin-fixed, paraffin-embedded
- ICI:
-
Immune checkpoint inhibitor
- NGS:
-
Next-genome sequencing
- NSCLC:
-
Non-small-cell lung cancer
- PD:
-
Progressive disease
- PR:
-
Partial response
- SD:
-
Stable disease
- TML:
-
Tumor mutational load
- TPS:
-
Tumor proportion score
References
Hurkmans D, Kuipers M, Smit J et al (2019) Combined assessment of tumour mutational load, CD8+ T cell infiltration, and expression of PD-L1 and HLA class I in small biopsy specimens to predict immunotherapy response in non-small cell lung cancer. Virchows Arch. 475:41 (OFP-10-008 Oral presentation)
Hurkmans D, Kuipers M, Smit J et al (2019) Tumor mutational load, CD8+ T cells, expression of PD-L1 and HLA class I to guide immunotherapy decisions. J Thoracic Oncol. 14:726 (P2.04-47 Poster abstract)
Sacher AG, Gandhi L (2016) Biomarkers for the clinical use of PD-1/PD-L1 Inhibitors in non-small-cell lung cancer: a review. JAMA Oncol 2:1217–1222. https://doi.org/10.1001/jamaoncol.2016.0639
Topalian SL, Taube JM, Anders RA, Pardoll DM (2016) Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 16:275–287. https://doi.org/10.1038/nrc.2016.36
Fujimoto D, Sato Y, Uehara K et al (2018) Predictive performance of four programmed cell death ligand 1 assay systems on nivolumab response in previously treated patients with non-small cell lung cancer. J Thorac Oncol 13:377–386. https://doi.org/10.1016/j.jtho.2017.11.123
Rizvi NA, Hellmann MD, Snyder A et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124–128. https://doi.org/10.1126/science.aaa1348
Chaudhary R, Quagliata L, Martin JP et al (2018) A scalable solution for tumor mutational burden from formalin-fixed, paraffin-embedded samples using the oncomine tumor mutation load assay. Transl Lung Cancer Res 7:616–630. https://doi.org/10.21037/tlcr.2018.08.01
Mehta AM, Jordanova ES, Kenter GG, Ferrone S, Fleuren GJ (2008) Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol Immunother 57:197–206. https://doi.org/10.1007/s00262-007-0362-8
McGranahan N, Rosenthal R, Hiley CT et al (2017) Allele-Specific HLA Loss and Immune Escape in Lung Cancer Evolution. Cell 171(1259–71):e11. https://doi.org/10.1016/j.cell.2017.10.001
Talebian Yazdi M, van Riet S, van Schadewijk A, Fiocco M, van Hall T, Taube C, Hiemstra PS, van der Burg SH (2016) The positive prognostic effect of stromal CD8+ tumor-infiltrating T cells is restrained by the expression of HLA-E in non-small cell lung carcinoma. Oncotarget 7:3477–3488. https://doi.org/10.18632/oncotarget.6506
Chowell D, Morris LGT, Grigg CM et al (2018) Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 359:582–587. https://doi.org/10.1126/science.aao4572
Ruiter DJ, Ferrier CM, van Muijen GN, Henzen-Logmans SC, Kennedy S, Kramer MD, Nielsen BS, Schmitt M (1998) Quality control of immunohistochemical evaluation of tumour-associated plasminogen activators and related components. European BIOMED-1 concerted action on clinical relevance of proteases in tumour invasion and metastasis. Eur J Cancer 34:1334–1340
Wang X, Li M (2019) Correlate tumor mutation burden with immune signatures in human cancers. BMC Immunol 20:4. https://doi.org/10.1186/s12865-018-0285-5
Rosenthal R, Cadieux EL, Salgado R et al (2019) Neoantigen-directed immune escape in lung cancer evolution. Nature 567:479–485. https://doi.org/10.1038/s41586-019-1032-7
Buck MD, Sowell RT, Kaech SM, Pearce EL (2017) Metabolic instruction of immunity. Cell 169:570–586. https://doi.org/10.1016/j.cell.2017.04.004
Gandara DR, Paul SM, Kowanetz M et al (2018) Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 24:1441–1448. https://doi.org/10.1038/s41591-018-0134-3
Li X, Guo F, Liu Y et al (2015) NLRC5 expression in tumors and its role as a negative prognostic indicator in stage III non-small-cell lung cancer patients. Oncol Lett 10:1533–1540. https://doi.org/10.3892/ol.2015.3471
Durgeau A, Virk Y, Gros G et al (2018) Human preprocalcitonin self-antigen generates TAP-dependent and -independent epitopes triggering optimised T-cell responses toward immune-escaped tumours. Nat Commun 9:5097. https://doi.org/10.1038/s41467-018-07603-1
Marijt KA, Van Der Burg SH, van Hall T (2019) TEIPP peptides: exploration of unTAPped cancer antigens. Oncoimmunology 8:1599639. https://doi.org/10.1080/2162402X.2019.1599639
Acknowledgements
The authors thank Annemarie van Schadewijk (Leiden University Medical Center, Leiden, The Netherlands) for help with the IHC analysis and Wesley van de Geer (Erasmus University Medical Center, Rotterdam, The Netherlands) for support with the cluster analysis.
Funding
This study was supported by an unrestricted grant from the Zabawas Foundation (J. Smit and P.S. Hiemstra) and an investigator-initiated study grant from Bristol-Myers Squibb (J.H. von der Thüsen, study identification number: OT123-361).
Author information
Authors and Affiliations
Contributions
DH, JS, PH, JA, SB and JT contributed to the conception and design of the study. DH drafted the manuscript. DH, PH, JA, JT and SB contributed to the acquisition, analysis, or interpretation of data. PP and RM aided in interpreting the results. JT, MK performed the histological examination of the samples. DH and JA performed radiological evaluation. MK, RvM, PH provided technical and material support. All authors have made critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
J.G.J.V. Aerts and P.E. Postmus have an advisory role for Bristol-Myers Squibb, J.H. von der Thüsen received research funding from Bristol-Myers Squibb. The authors declare that there are no other conflicts of interest.
Ethical approval and ethical standards
Trial number and trial registration details: MULTOMAB trial, Dutch Trial Register number NTR7015/ NL6828, ethics approval by responsible authority Medical Research Ethics Committee Erasmus MC, reference number: 16-011. The use of archival FFPE samples was in accordance with guidelines from the Dutch Federation of Medical Research and was approved by responsible authority Medical Research Ethics Committee Erasmus MC, reference number: 17-1186.
Informed consent
All patients signed written informed consent to use data and materials for research and publication, which was provided before study entry (MULTOMAB trial, NTR7015/NL6828).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jan H. von der Thüsen and Sjoerd H. van der Burg Shared senior authorship
Parts of the work have been presented during a conference: European Society for Medical Oncology (ESMO) Congress, September 29th, Barcelona, Spain (published as poster) and ECP 2019, September 10th, Nice, France (oral presentation) [1, 2].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hurkmans, D.P., Kuipers, M.E., Smit, J. et al. Tumor mutational load, CD8+ T cells, expression of PD-L1 and HLA class I to guide immunotherapy decisions in NSCLC patients. Cancer Immunol Immunother 69, 771–777 (2020). https://doi.org/10.1007/s00262-020-02506-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02506-x