Abstract
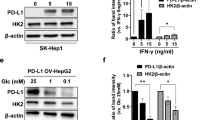
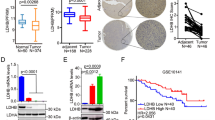
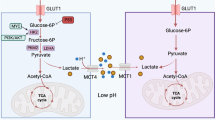
From a metabolic perspective, cancer may be considered as a metabolic disease characterized by reprogrammed glycolytic metabolism. The aim of the present study was to investigate CD147-mediated glucose metabolic regulation in hepatocellular carcinoma (HCC) and its contribution to altered immune responses in the tumor microenvironment. Several HCC cell lines and corresponding nude mice xenografts models differing in CD147 expressions were established to directly investigate the role of CD147 in the reprogramming of glucose metabolism, and to determine the underlying molecular mechanisms. Immunohistochemistry (IHC) analyses and flow cytometry were used to identify the relationship between reprogrammed glycolysis and immunosuppression in HCC. Upregulated CD147 expressions were found to be associated with enhanced expressions of GLUT1, MCT1 in HCC tumorous tissues. CD147 promoted the glycolytic metabolism in HCC cell lines in vitro via the PI3K/Akt/mTOR signaling pathway. A positive correlation existed between a profile of immunosuppressive lymphocytes infiltration and CD147 expression in HCC tissues. Accumulation of FOXP3-expressing regulatory T cells was induced under a stimulation with lactate in vitro. In conclusion, CD147 promoted glycolytic metabolism in HCC via the PI3K/Akt/mTOR signaling pathway, and was related to immunosuppression in HCC.





Similar content being viewed by others
Abbreviations
- CTL:
-
Cytotoxic T lymphocyte
- CTLA-4:
-
Cytotoxic T lymphocytes associated protein-4
- DAB:
-
Diaminobenzidine buffer
- DC:
-
Dendritic cell
- 2-DG:
-
2-Deoxy-d-glucose
- DMEM:
-
Dulbecco’s modified eagle medium
- ECL:
-
Enhanced chemiluminescence
- EGFP:
-
Enhanced green fluorescent protein
- EMMPRIN:
-
Extracellular matrix metalloproteinase inducer
- FACS:
-
Fluorescence Activated Cell Sorting
- FCS:
-
Fetal calf serum
- 18F-FDG:
-
18F-fluorodeoxyglucose
- FOXP3:
-
Forkhead box protein 3
- GLUT1:
-
Glucose transporter 1
- HBs Ag:
-
Hepatitis B surface antigen
- HCC:
-
Hepatocellular carcinoma
- HCV Ab:
-
Hepatitis C virus antibody
- HRP:
-
Horseradish peroxidase
- IHC:
-
Immunohistochemistry
- IL-2:
-
Interleukin 2
- LDH:
-
Lactate dehydrogenase
- MCT1/4:
-
Monocarboxylate transporters 1 and 4
- MNC:
-
Mononuclear cell
- MOI:
-
Multiplicity of transduction
- mTOR:
-
Mammalian target of rapamycin
- NIL:
-
Non-tumor-infiltrating lymphocytes
- NK:
-
Natural killer
- NT:
-
Natural T
- OCR:
-
Oxygen consumption
- PBMC:
-
Peripheral blood mononuclear cell
- PBS:
-
Phosphate buffer solution
- PD-1:
-
Programmed cell death-1
- PET/CT:
-
Positron Emission Tomography/Computed Tomography
- PI3K:
-
Phosphatidylinositol 3-kinase
- PVDF:
-
Polyvinylidene difluoride membranes
- RIPA:
-
Radio-Immunoprecipitation Assay
- RPMI:
-
Roswell Park Memorial Institute
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- ShRNA:
-
Small hairpin RNA
- SPSS:
-
Statistical software package
- SUVmax :
-
Maximal standard uptake value
- TBS:
-
Tris buffer saline
- Teff:
-
Effector T cell
- TGF-β1:
-
Transforming growth factor
- TIL:
-
Tumor-infiltrating lymphocytes
- TNM:
-
Tumor Node Metastasis
References
Lafaro KJ, Demirjian AN, Pawlik TM (2015) Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am 24:1–17
Sangro B, Gomez-Martin C, de la Mata M et al (2013) A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 59:81–88
Liu X, Qin S (2019) Immune checkpoint inhibitors in hepatocellular carcinoma: opportunities and challenges. Oncologist 24:S3–S10
Floudas CS, Brar G, Greten TF (2019) Immunotherapy: current status and future perspectives. Dig Dis Sci 64:1030–1040
Li X, Peng J, Pang Y et al (2015) Identification of a FOXP3(+)CD3(+)CD56(+) population with immunosuppressive function in cancer tissues of human hepatocellular carcinoma. Sci Rep 5:14757
Speiser DE, Ho PC, Verdeil G (2016) Regulatory circuits of T cell function in cancer. Nat Rev Immunol 16:599–611
Renner K, Singer K, Koehl GE et al (2017) Metabolic Hallmarks of tumor and immune cells in the tumor microenvironment. Front Immunol 8:248
Kroemer G, Pouyssegur J (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13:472–482
Hsu PP, Sabatini DM (2008) Cancer cell metabolism: warburg and beyond. Cell 134:703–707
Villalba M, Rathore MG, Lopez-Royuela N, Krzywinska E, Garaude J, Allende-Vega N (2013) From tumor cell metabolism to tumor immune escape. Int J Biochem Cell Biol 45:106–113
Chang CH, Qiu J, O’Sullivan D et al (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162:1229–1241
MacIver NJ, Michalek RD, Rathmell JC (2013) Metabolic regulation of T lymphocytes. Annu Rev Immunol 31:259–283
Siska PJ, Rathmell JC (2015) T Cell metabolic fitness in anti-tumor immunity. Trends Immunol 36:257–264
Domblides C, Lartigue L, Faustin B (2019) Control of the antitumor immune response by cancer metabolism. Cells 8(pii):E104
Brand A, Singer K, Koehl GE et al (2016) LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK Cells. Cell Metab 24:657–671
Scott KE, Cleveland JL (2016) Lactate wreaks havoc on tumor-infiltrating T and NK cells. Cell Metab 24:649–650
Zhao E, Maj T, Kryczek I et al (2016) Cancer mediates effector T cell dysfunction by targeting micro RNAs and EZH2 via glycolysis restriction. Nat Immunol 17:95–103
Xin X, Zeng X, Gu H et al (2016) CD147/EMMPRIN overexpression and prognosis in cancer: a systematic review and meta-analysis. Sci Rep 6:32804
Peng F, Li H, You Q et al (2017) CD147 as a novel prognostic biomarker for hepatocellular carcinoma: a meta-analysis. Biomed Res Int 2017:5019367
Li X, Yu X, Dai D, Song X, Xu W (2016) The altered glucose metabolism in tumor and a tumor acidic microenvironment associated with extracellular matrix metalloproteinase inducer and monocarboxylate transporters. Oncotarget 7:23141–23155
Ke X, Fei F, Chen Y et al (2012) Hypoxia upregulates CD147 through a combined effect of HIF-1α and Sp1 to promote glycolysis and tumor progression in epithelial solid tumors. Carcinogenesis 33:1598–1607
Huang Q, Li J, Xing J et al (2014) CD147 promotes reprogramming of glucose metabolism and cell proliferation in HCC cells by inhibiting the p53-dependent signaling pathway. J Hepatol 61:859–866
Dai JY, Dou KF, Wang CH et al (2009) The interaction of HAb18G/CD147 with integrin alpha6beta1 and its implications for the invasion potential of human hepatoma cells. BMC Cancer 9:337
Zhao P, Zhang W, Tang J et al (2010) Annexin II promotes invasion and migration of human hepatocellular carcinoma cells in vitro via its interaction with HAb18G/CD147. Cancer Sci 101:387–395
Yeung SJ, Pan J, Lee MH (2008) Roles of p53, MYC and HIF-1 in regulating glycolysis—the seventh hallmark of cancer. Cell Mol Life Sci 65:3981–3999
Zheng YL, Li L, Jia YX et al (2019) LINC01554-mediated glucose metabolism reprogramming suppresses tumorigenicity in hepatocellular carcinoma via downregulating PKM2 expression and inhibiting Akt/mTOR signaling pathway. Theranostics 9:796–810
Li X, Fu Q, Zhu Y et al (2019) CD147-mediated glucose metabolic regulation contributes to the predictive role of 18 F-FDG PET/CT imaging for EGFR-TKI treatment sensitivity in NSCLC. Mol Carcinog 58:247–257
Gou X, Tang X, Kong DK et al (2016) CD147 is increased in HCC cells under starvation and reduces cell death through upregulating p-mTOR in vitro. Apoptosis 21:110–119
Ganapathy-Kanniappan S (2017) Linking tumor glycolysis and immune evasion in cancer: emerging concepts and therapeutic opportunities. Biochim Biophys Acta Rev Cancer 1868:212–220
Ganapathy-Kanniappan S (2017) Taming tumor glycolysis and potential implications for immunotherapy. Front Oncol 7:36
Cham CM, Driessens G, O’Keefe JP, Gajewski TF (2008) Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur J Immunol 38:2438–2450
San-Millán I, Brooks GA (2017) Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 38:119–133
Hirschhaeuser F, Sattler UG, Mueller-Klieser W (2011) Lactate: a metabolic key player in cancer. Cancer Res 71:6921–6925
Romero-Garcia S, Moreno-Altamirano MM, Prado-Garcia H, Sánchez-García FJ (2016) Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front Immunol 7:52
Feng J, Yang H, Zhang Y et al (2017) Tumor cell-derived lactate induces TAZ dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene 36:5829–5839
Caronni N, Simoncello F, Stafetta F et al (2018) Downregulation of membrane trafficking proteins and lactate conditioning determine loss of dendritic cell function in lung cancer. Cancer Res 78:1685–1699
Harmon C, Robinson MW, Hand F et al (2019) Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol Res 7:335–346
Long Y, Gao Z, Hu X et al (2018) Downregulation of MCT4 for lactate exchange promotes the cytotoxicity of NK cells in breast carcinoma. Cancer Med 7:4690–4700
Fischer K, Hoffmann P, Voelkl S et al (2007) Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109:3812–3819
Angelin A, Gil-de-Gómez L, Dahiya S et al (2017) FOXP3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab 25:1282–1293
Acknowledgements
We would like to thank Wenwen Yu at Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) for her assistance in FACS analyses.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81601377, 81501984 and 2018ZX09201015), the Tianjin Natural Science Fund (Grant Nos. 16JCZDJC35200, 17JCYBJC25100, 18PTZWHZ00100 and H2018206600), the Science and Technology Development Fund of Tianjin Education Commission for Higher Education (Grant Nos. 2018KJ057 and 2018KJ061), the Tianjin Medical University Cancer Institute and Hospital Fund (Grant Nos. Y1601, B1605, B1719, Y1805 and Y1810) and the Incubation Project of the National Clinical Research Center for Cancer (Grant No. N14B09).
Author information
Authors and Affiliations
Contributions
XFL and YFZ contributed to the experimental design, the operation of the experiments, data analyses and interpretation of the results in this study. They both contributed to drafting the manuscript. WCM performed the statistical analyses and drafted the manuscript. QF and JJL performed the cellular and immunological experiments. GTY and PHC established the nude mice xenograft models and performed the imaging tests in vivo. DD and WC designed the study and interpreted the results. LSQ and XZY designed the study and reviewed the draft of the manuscript. WGX was responsible for data interpretation and assessing the final content of the manuscript. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Ethical approval and ethical standards
All procedures were performed in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The present study was approved by Hospital Ethic Review Committee of Peking University People’s Hospital (Beijing, China) on June 6, 2017. All protocols involving animals were in strict accordance with institutional guidelines for the care and use of experimental animals and were approved by the animal ethical review committee of Tianjin Medical University and Cancer Institute (Tianjin, China) on August 18, 2018.
Informed consent
All patients provided written informed consent before surgery for use of their specimens for research. Oral informed consent was obtained from the healthy donors at the Tianjin Blood Center to the use of their blood for research purposes.
Animal source
Female BALB/c nude mice (Beijing Vital River Laboratory Animal Technology Co., Ltd) with an average body weight of 18–22 g were purchased and maintained in specific pathogen-free condition for use.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Zhang, Y., Ma, W. et al. Enhanced glucose metabolism mediated by CD147 contributes to immunosuppression in hepatocellular carcinoma. Cancer Immunol Immunother 69, 535–548 (2020). https://doi.org/10.1007/s00262-019-02457-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02457-y