Abstract
Objectives
Patients with human papillomavirus (HPV)-positive oropharyngeal squamous cell carcinomas (OPSCCs) have an improved prognosis compared to HPV-negative OPSCCs. Several theories have been proposed to explain this relatively good prognosis. One hypothesis is a difference in immune response. In this study, we compared tumor-infiltrating CD3+, CD4+, CD8+ T-cells, and granzyme inhibitors (SERPINB1, SERPINB4, and SERPINB9) between HPV-positive and HPV-negative tumors and the relation with survival.
Methods
Protein expression of tumor-infiltrating lymphocytes (TILs) (CD3, CD4, and CD8) and granzyme inhibitors was analyzed in 262 OPSCCs by immunohistochemistry (IHC). Most patients (67 %) received primary radiotherapy with or without chemotherapy. Cox regression analysis was carried out to compare overall survival (OS) of patients with low and high TIL infiltration and expression of granzyme inhibitors.
Results
HPV-positive OPSCCs were significantly more heavily infiltrated by TILs (p < 0.001) compared to HPV-negative OPSCCs. A high level of CD3+ TILs was correlated with a favorable outcome in the total cohort and in HPV-positive OPSCCs, while it reached no significance in HPV-negative OPSCCs. There was expression of all three granzyme inhibitors in OPSCCs. No differences in expression were found between HPV-positive and HPV-negative OPSCCs. Within the group of HPV-positive tumors, a high expression of SERPINB1 was associated with a significantly worse overall survival.
Conclusion
HPV-positive OPSCCs with a low count of CD3+ TILs or high expression of SERPINB1 have a worse OS, comparable with HPV-negative OPSCCs. This suggests that the immune system plays an important role in the carcinogenesis of the virally induced oropharynx tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The causative role of the human papillomavirus (HPV), especially in oropharynx squamous cell carcinoma (OPSCC), is widely established [1]. Studies showed prevalence rates varying between 15 and 90 % of HPV-positive tumors in OPSCC worldwide and an increase is reported in the last two decades [2, 3]. Despite their general presentation at a more advanced stage, HPV-positive OPSCCs have a better survival compared to HPV-negative OPSCCs [4, 5].
The exact cause of this survival advantage is not fully elucidated and seems to be related to multiple factors such as the absence of field cancerization and wild-type expression of p53 and Rb [6]. Additionally, non-oropharyngeal HPV-positive head and neck squamous cell carcinoma (HNSCC) does not show this improved outcome compared to their HPV-negative counterparts [7]. This observation suggests that the behavior of a HNSCC induced by HPV might be influenced by the tissue microenvironment of that specific subsite. The subepithelial tissue in the oropharynx is mainly lymphoid, indicating that especially the immune system might harbor an important clue in the different behavior of HPV-positive tumors in the oropharynx.
For many cancers, it has become clear that in addition to genetic and epigenetic changes in the neoplastic cells, also microenvironmental factors play an important role in carcinogenesis and tumor progression. Cancers can survive the host immune response by escaping recognition and suppression of the host immune system [8]. Indeed, several studies show an increased immune response in HPV-positive OPSCC in the primary tumor which may contribute to their improved treatment response [9–11].
Recognition of the important regulatory role of the immune system on the behavior of cancer cells has led to the awareness that manipulating the microenvironment could be used as therapeutic target. Indeed, stimulating the immune response against the cancer cells has been shown very successful in various cancer types [12, 13]. However, effective immunotherapy can only be achieved when potential mechanisms of the tumor to escape from recognition or killing by the immune system are overcome. Moreover, expression of intracellular serine protease inhibitors (SERPINS) by tumors could help the tumor cells to escape from granzyme-mediated apoptosis pathways and can lead to a poor response to cellular immunotherapy [8]. This pathway is the major mechanism via which cytotoxic lymphocytes eliminate virus-infected and tumor cells by releasing granules containing serine proteases (granzymes) to induce apoptosis [14, 15]. Humans express five different granzymes ((Gr) GrA, GrB, GrK, GrH, and GrM)) and intracellular inhibitors for GrB, GrM, and GrH have been identified, being SERPINB9, SERPINB4, and SERPINB1, respectively [16–19].
We investigated the intratumoral immune cell response, as well as the expression of these intracellular granzyme inhibitors in OPSCC as a potential mechanism for tumor cell immune evasion and correlated their expression with clinicopathological variables such as HPV status and overall survival.
Materials and methods
Patient selection and clinicopathological information
Our study cohort involved 288 patients with histologically proven primary OPSCC curatively treated at the University Medical Center Utrecht, the Netherlands, between 1997 and 2011 of which formalin fixed paraffin-embedded (FFPE) tissue blocks of the primary tumor were available in our pathology archives. The tissue blocks were incisional biopsies, as the treatment was mostly primary radiotherapy or chemoradiotherapy.
Since the tissue used was a remainder following the clinical diagnostic process, no ethical approval was required according to Dutch national ethical guidelines (www.federa.org). Anonymous or coded use of leftover tissue for scientific purposes is part of standard treatment agreement with patients in our center [20].
Tissue microarrays were constructed as described previously [21]. Briefly, hematoxylin–eosin-stained slides were examined and revised by a dedicated head and neck pathologist (S. M. Willems) to mark representative tumor regions. Four tissue microarrays (TMA) were constructed using a fully automated tissue microarray instrument (TMA Grand Master, Beecher Instruments). From each tumor block, three cores with a diameter of 0.6 mm were punched out and placed in a recipient paraffin block. Areas including necrosis were avoided. In addition, normal oropharynx mucosa obtained from patients with neck metastasis from an unknown primary tumor was incorporated in each block as a control among the different TMAs. Nowadays, TMAs are accepted as a fast and accurate approach for the evaluation of immunohistochemical stainings in large groups [22].
From all included OPSCCs, patient characteristics, clinical TNM classification (based on palpation, ultrasound-guided fine-needle aspiration, MRI or CT, and classified in a multidisciplinary panel) based on the AJCC TNM classification of malignant tumors 7th edition and survival data were extracted from medical records. HPV status was determined for all tumors by a combination of p16 immunohistochemistry and linear array, as described earlier [21]. Adequate DNA for HPV testing and enough percentage tumor cells (>30 %) for constructing a TMA was available for 262 cases of the initial 288 OPSCC cohort (91 %).
Immunohistochemistry
Immunohistochemical evaluation of tumor-infiltrating lymphocytes, GrB, and the three granzyme inhibitors (SERPINB1, SERPINB4, and SERPINB9) was performed on 4 µm paraffin TMA slides. Sections were heated at 60 °C overnight. All tissue sections were deparaffinized with xylene and rehydrated in decreasing ethanol dilutions. Endogenous peroxidase activity was blocked for 15 min in a 0.3 % hydrogen peroxidase phosphate-citrate buffer (pH 5.8) followed by antigen retrieval. For CD3+, CD4+, CD8+, granzyme B, and SERPINB1, IHC staining was performed using an automated immunostainer (Benchmark Ultra, Ventana, Roche). All other stainings were performed manually. After antigen retrieval by boiling the sections in EDTA buffer pH 9.0 or citrate buffer pH 6.0 for 20 min, sections were cooled within the buffers for 30 min followed by incubation with the primary antibodies for 60 min. The used antibodies, antigen retrieval, and appropriate dilutions are summarized in Table 1. For the detection of the primary antibodies, a poly-HRP anti-mouse, rabbit, rat (ready to use: Brightvision, DPVO-HRP, immunologic), or in case of SERPINB4, the Novolink kit (Leica) was used according to manufacturer’s protocol. Between steps, slides were washed three times with PBS. Slides were then developed with diaminobenzidine for 10 min followed by counterstaining with hematoxylin, dehydration in alcohol and mounting. Finally, the sections were coverslipped. Appropriate negative and positive controls were used in all stainings.
Evaluation of immunohistochemical staining
Scoring of TMA cores was performed mutually by a dedicated head and neck pathologist (S. M. Willems) and a researcher (P. M. W. van Kempen), both blinded to clinical characteristics of the patients. A core was excluded when >95 % of the core contained no tissue or when the core contained <5 % tumor tissue. Discordance was resolved by discussion and consonance. Patients were only included in the analyses if one or more tumor cores were available. For CD3+, CD4+, and CD8+, the number of positively stained lymphocytes infiltrating the tumor tissue was counted. For SERPINB1 and SERPINB4, the percentages of nuclear or cytoplasmic stained tumor cells were scored. For SERPINB4, staining intensity was scored as well and evaluated as absent (0), weak (1), moderate (2), and strong (4). SERPINB9 was scored as positive (staining in more than 10 % of tumor cells) or negative as used in previous studies [23]. We investigated the expression of SERPINS in neoplastic epithelial cells, although SERPINS are also expressed in other cell types, e.g., lymphocytes and fibroblasts where they exhibit specific functions [24].
When more than one core was available for one case, the mean number (CD3+, CD4+, CD8+) or the mean percentage (SERPINB1, SERPINB4) was calculated. For SERPINB4, a H-score was calculated by multiplying the mean percentage of SERPINB4 staining with the maximum staining intensity. For all these proteins, an optimized cutoff point with regard to overall survival was identified using ROC curves. For CD3+, the optimal cutoff value was 150, and for CD4+ and CD8+, it was 100. For SERPINB1 and SERPINB4, it both was 50 %, and using the H-score for SERPINB4, the threshold was identified at 150. The activity of cytotoxic tumor-infiltrating lymphocytes was examined by GrB expression. Per tumor core, the number of GrB-positive cells were counted and divided by the number of cytotoxic lymphocytes.
Statistical analyses
SPSS IBM, version 22.0, was used for all analyses. Differences in patient characteristics between HPV-positive and HPV-negative OPSCCs were calculated using the Pearson’s chi-squared test for categorical variables and the student’s t test for normally distributed continuous variables. The Mann–Whitney U test was used to assess differences in the expression of CD3+, CD4+, CD8+, SERPINB1, and SERPINB4 between the HPV-positive and HPV-negative OPSCCs as these continuous variables were non-normally distributed. Correlations were analyzed using Pearson correlation. Overall survival (OS) was used as outcome endpoint and defined as the time between date of first tumor positive biopsy and death. Censored patients were confirmed alive at time of censoring. Thus, there was no loss to follow-up. Survival rates were plotted according to the Kaplan–Meier method, and associations were analyzed using the log-rank test. Effect modification and confounding were investigated. Multivariate analyses were performed using the Cox proportional hazard model in a stepwise-backward selection procedure including the variables that were significant in univariate analysis. Receiver operating characteristic (ROC) curves analyses were used to determine cutoff points for protein expression and survival. Statistical significance was set at p < 0.05.
Results
Patients’ characteristics
The study included 262 OPSCC patients with a mean age of 59 at primary diagnosis. Overall, 17 % of the OPSCCs were HPV-positive and 83 % were HPV-negative. Patient and tumor characteristics are depicted in Table 2. In line with the literature, patients with HPV-negative tumors had higher T-stages compared to patients with HPV-positive tumors, but there were fewer cases of nodal metastases. With regard to smoking and alcohol abuse, patients with HPV-positive tumors used significant less alcohol. There was no significant difference between the groups considering smoking. Cisplatinum-based chemoradiotherapy (CCRT) was the main treatment of patients (Table 2). CCRT was administered to patients below 70 years of age, if tumors were functionally or technically inoperable (T3–T4 tumors) and if there were no further contra-indications to undergo this treatment.
HPV-status and tumor infiltration by immune cells
Due to insufficient tissue, a few cases could not be evaluated for CD3+, CD4+, or CD8+ staining (Table 2). The number of intratumoral CD3+, CD4+, and CD8+ cells was assessed and compared between HPV-positive and HPV-negative OPSCCs (Fig. 1a). The mean number of intratumor CD3+, CD4+, and CD8+ cells was significantly higher in HPV-positive tumors. The number of tumor-infiltrating CD3+, CD4+, and CD8+ positive cells was dichotomized (CD3+ with a cutoff value of 150; CD8+ and CD4+ with a cutoff value of 100) and compared between HPV-positive and HPV-negative tumors. A high number (>150) of tumor-infiltrating CD3+ cells was observed in 28 of 214 (13 %) HPV-negative tumors compared to 25 of 43 (58 %) HPV-positive tumors (p < 0.001). Furthermore, a high number intratumor CD4+ and CD8+ positive cells (>100) was found in 41 of 213 (19 %) and 31 of 208 (15 %) HPV-negative tumors versus 21 of 43 (49 %) and 23 of 39 (59 %) HPV-positive tumors. These differences were significant (p < 0.001).
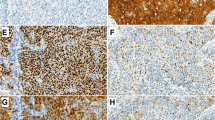
a Staining patterns of CD3+, CD4+, and CD8+. Example of a 0.6 mm TMA core with low expression on the left side (A) and high expression on the right side for CD3+ (B) (magnification 10× and magnification 20×). The same for CD4+ (C, D) and CD8+ (E, F). b Staining patterns of granzyme B, SERPINB1, SERPINB4, and SERPINB9. Example of a 0.6 mm TMA core with low expression on the left side compared to high expression on the right side for granzyme B (A, B), SERPINB1 (C, D), SERPINB4 (E, F), and SERPINB9 (G, H) (magnification 10× and magnification 20×)
Expression of granzymes inhibitors
In normal tissue of the oropharyngeal region SERPINB9, SERPINB1, and SERPINB4, expression was absent. For SERPINB9, SERPINB1, and SERPINB4, we mainly observed a cytoplasmic staining with sometimes nuclear staining as well (Fig. 1b). The proportion of patients with positive and negative SERPIN staining is depicted in Table 2. Even though SERPIN expression was in all cases lower in HPV-positive tumors than in HPV-negative tumors, none of these differences reached statistical significance. SERPINB9 expression could be scored in 235 OPSCCs (90 %). The expression level of SERPINB9 was heterogeneous and positive in 28 (12 %) of 235 evaluable cases. There was no significant difference in SERPINB9 expression between HPV-positive and HPV-negative tumors. SERPINB1 and SERPINB4 expression could be scored in 242 (92 %) and 243 (93 %) of the cases, respectively. Using a cutoff value of 50 %, 71 tumors of the 242 (29 %) showed a high expression of SERPBINB1 in the total cohort. The HPV-positive OPSCC showed a high expression of SERPINB1 in 25 % (10 of 40) compared to 30 % (61 of 202) of HPV-negative, though this difference was not significant (p = 0.639). SERPINB4 staining intensity, as well as the percentage of SERPINB4 positive tumor cells, was compared between HPV-positive and HPV-negative OPSCC. The SERPINB4 intensity, dichotomized as absent (0)/weak (1) versus moderate (2)/strong (3), was observed as high (moderate/strong) in 18 of 39 (46 %) of HPV-positive OPSCC compared to 120 of 204 (59 %) HPV-negative tumors (p = 0.564). Using a cutoff value of 50 %, 20 of 39 (51 %) HPV-positive compared to 124 of 204 (61 %) HPV-negative tumors showed a high SERPINB4 expression (p = 0.353). Results are summarized in Table 2.
We correlated expression of intratumoral CD3+, CD4+, and CD8+ to expression of the granzyme inhibitors. There was no significant correlation between expression of TIL’s and granzyme inhibitors. GrB expression was evaluated to determine the activity of cytotoxic tumor-infiltrating lymphocytes. Tumors that showed SERPINB9 expression (>10 %) contained a significant higher percentage of granzyme B-positive cytotoxic T lymphocytes (CTLs) (mean granzyme B-positive CTLs 23 vs. 38 % in SERPINB9 positive tumors).
Effect of tumor infiltration by immune cells and granzyme inhibitors on survival
Univariate analyses using Kaplan–Meier curves were performed to evaluate factors potentially associated with OS. The analyses were performed in the total cohort, as well as stratified for patients with HPV-positive and HPV-negative tumors The median follow-up was 35 months for all patients (interquartile range [IQR]: 15.8–67.0). Of the total cohort of 274 patients, 151 (55 %) patients had died at the end of follow-up.
Prognostic factors included in the univariate analysis were as follows: age, gender, tumor size (cT1-2 vs. cT3-4), smoking, alcohol use, nodal stage (cN0 vs. cN+), number of intratumor CD3+, CD8+, and CD4+ cells, SERPINB9 staining (positive vs. negative), SERPINB1 staining (high vs. low expression), and SERPINB4 staining (H-score high vs. low expression). In the total group, HPV-positivity, small tumor size (T1–T2), no lymph node metastasis, high number of CD3+ and CD8+ intratumor cells, and a low expression of SERPINB1 in tumor cells were all individually significant associated with an improved OS (Table 3, Fig. 2). The Cox proportional hazard regression model showed that tumor size (T1–T2), nodal stage (N0), HPV positivity, and a high number of intratumor CD3+ cells were independent prognostic factors for an improved OS (Table 4).
Kaplan–Meier curves for OPSCC overall survival. Kaplan–Meier curves of OS. a For high levels (>150) of intratumoral CD3+ cells versus low levels in the total cohort of OPSCC. Log-rank p < 0.001, hazard ratio = 0.36 (0.21–0.61), p < 0.001. b For HPV-positive tumors. Log-rank p = 0.029, hazard ratio = 0.29 (0.09–0.95), p = 0.042 c For HPV-negative tumors. Log-rank p = 0.060, hazard ratio = 0.61 (0.37–1.03), p = 0.065. d Kaplan–Meier OS curve for SERPINB1 expression stratified for HPV status. It shows that the OS of HPV-positive tumors with a high SERPINB1 expression is comparable to HPV-negative patients
In HPV-negative patients, tumor size and nodal stage were correlated with OS in univariate analysis. Neither number of infiltration of T-cells nor SERPINB1 expression was of significant importance. In HPV-positive tumors, a high number of intratumoral CD3+ cells, low expression of SERPINB1, and younger age were associated with a significant better OS (Table 3; Fig. 2). As the group of HPV-positive patients was rather small and only 10 deaths occurred in this subgroup, multivariate analysis was not possible. In HPV-negative patients, multivariate analysis showed that a high T- or N-stage were independently associated with a worse OS, while CD3+ cell influx was not (Table 4).
Discussion
Previous studies showed that the immune system is involved in the process of carcinogenesis of HNSCC and elevated levels of tumor-infiltrating lymphocytes (TILs) are associated with an improved survival [9, 25]. Considering the poor survival rates, HNSCC patients may therefore benefit from additional immunotherapy in the future. In this study, we evaluated the amount of CD3+, CD4+, CD8+ TILs, and expression of granzyme inhibitors SERPINB1, SERPINB4, and SERPINB9 in relation to HPV-status and survival in OPSCC. In our study, HPV-positive tumors showed to have a significantly higher number of CD3+, CD4+, and CD8+ TILs. A high number of CD3+ TILs correlated with improved survival, independent of HPV status. In addition, we show for the first time that OPSCCs express granzyme inhibitors (SERPINB1, SERPINB4, and SERPINB9) to evade granzyme-induced cytotoxicity. Their expression was not different between HPV-positive and HPV-negative tumors. However, a high SERPINB1 expression identified a subgroup of HPV-positive tumors with a poor survival, which is comparable to HPV-negative tumors.
In OPSCC, HPV status is a prognostic biomarker as HPV-positive tumors have a better long-term survival compared to HPV-negative OPSCC [1]. Moreover, HPV-positive OPSCC represents a distinct entity with different tumor biology reflected on genetic and epigenetic levels [26, 27]. We hypothesized that the immune response and expression of granzyme inhibitors may also differ between HPV-positive and HPV-negative tumors. In our study, the immune profiles of HPV-positive and HPV-negative OPSCC were distinct, notable for differences in levels of CD3+, CD8+, and CD4+ TILs. This suggests that viral proteins released by HPV-positive tumors might be presented in MHC class I and II molecules on the surface and elicit a strong immune response after recognition by CD4+ and CD8+ T-cells. This pro-immunogenic effect of HPV triggers an anti-tumorigenic immune response, which might partly explain the improved survival in this group. These findings are in line with the previous studies, although not in all studies the differences were statistically significant [9–11, 28–34]. This could be explained by the variation in HPV detection methods, difference in used antibodies for assessing of intratumoral lymphocytes and used cutoff values.
As HPV-positive patients have an improved survival compared to those with HPV-negative OPSCC, it could be that less intensive treatment regimens in HPV-positive tumors could achieve similar efficacy with less side effects. Therefore, several clinical trials are currently investigating different ways to de-escalate treatment and still receive local control [35]. However, several studies showed that not all HPV-positive OPSCC have an excellent survival [4, 36]. Gillison et al. [36] showed that heavy smoking patients with a HPV-positive tumor have reduced survival, almost comparable to patients with HPV-negative tumors. Additionally, Ward et al. [9] developed a prognostic model suggesting that patients with HPV-positive tumors with low levels of tumor-infiltrating lymphocytes have similar survival rates to HPV-negatives. This is in line with the findings in our cohort showing that elevated levels of CD3+ cells are an independent predictor for improved survival of OPSCC comparable in strength to HPV status as prognosticator. A stratified analysis in HPV-positive and HPV-negative patients showed that an increased influx of CD3+ cells in HPV-positive patients is significant associated with an improved survival. In contrast, in HPV-negative patients, this association reached no significance. These results suggest that the immune system plays a more important role in the clinical behavior of HPV-positive OPSCC than in HPV-negative OPSCC.
SERPIN family members play pivotal roles in the inhibition of serine proteases in blood coagulation, mental diseases, and innate immune responses [37]. Studies with knock out of SERPINB1 in mice showed a significant role for SERPINB1 in protecting lung antimicrobial proteins from proteolysis during microbe infection and its regulatory role in sustaining the balance of neutrophil reserve [38, 39]. Increasing evidence shows that granzyme H participates in the defense of inflammation and SERPINB1 is identified as a possible inhibitor [19]. This study identified that a high expression of SERPINB1 is associated with a worse prognosis in HPV-positive patients and could be a mechanism of the tumor to escape from recognition or killing by the immune system. A possible explanation is that the immunogenic response is higher in HPV-positive OPSCC, and therefore, the effect of granzyme inhibition is more effective in HPV-positive tumors. Alternatively, Tseng et al. [40] showed that SERPINB1 might have a role in promoting motility in oral cancer cells and increasing invasiveness. This indicates a correlation with tumor metastases and therefore a worse prognosis.
In selecting patients properly for immunotherapy, it is important to account for the presence of suppressive mechanisms of immune system. The granule exocytosis pathway is the major mechanism via which cytotoxic lymphocytes, including natural killer (NK) cells and cytotoxic T-lymphocytes, counteract virus-infected cells and tumor tissue. The principal mechanism of killing in this pathway is by the release of granules containing the pore-forming protein perforin and a family of structurally homologues serine proteases known as granzymes into the immunological synapse. Pore formation induced by perforin facilitates the introduction of granzymes into the cell. In the cell, they can induce apoptosis by the cleavage of intracellular substrates [14, 41]. Humans express five different granzymes: granzyme A, B, H, K, and M [16]. All these granzymes can induce cell death, although each granzyme induces a unique pathway that only overlaps partially. The balance between activation and inhibition of the proteolytic cascade must be tightly controlled to avoid self-damage by, for example, intracellular leakage of granzymes. Additionally, tumor and virus-infected cells use strategies to evade granzyme induced cell death. It has been well established that granzyme B activity is controlled by SERPINB9 [42]. Recently, two other granzyme inhibitors were identified. SERPINB1 has been identified as a granzyme H inhibitor and SERPINB4 as a granzyme M inhibitor [17, 19]. Therefore, we assessed the expression of these three granzyme inhibitors. Multiple studies show that expression of SERPINB9 in melanomas is correlated with worse survival and poor response to immunotherapy [43]. For HNSCC, the expression of granzyme inhibitors has not been studied yet. In this study, we show for the first time that SERPINB9, SERPINB1, and SERPINB4 are not expressed in normal tissue, but expression can be acquired in OPSCC. There was no difference in expression of the studied SERPINS between HPV-positive and HPV-negative tumors. No correlation was found between the expression of SERPINS and the number of TILs. The observation of expression of granzyme inhibitors in OPSCC is important as it may affect the success rate of immunotherapy in OPSCC.
The results of study should be viewed within the constraints of several limitations. First, TMA’s were used for the evaluation of the IHC stainings. Although TMA is accepted nowadays as a fast and accurate approach for the evaluation of IHC stainings in large groups, it must be denoted that it could introduce possible bias due to the high heterogeneity of HNSCC. Second, overexpression of SERPINB1 in HPV-positive OPSCC showed a significant association with worse survival, but the low number of patients and events (N = 10) in the HPV-positive group did not allow for multivariate analysis. Therefore, our results should be validated in a cohort with higher numbers of HPV-positive patients, and analyses should be stratified for therapy.
In conclusion, it is plausible that the immune system plays a more important role in carcinogenesis of virally induced oropharynx tumors. Consequently, HPV-positive patients with a high expression of SERPINB1 or low invasion of CD3+ cells should receive additional therapy instead of treatment de-escalation.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- CCRT:
-
Cisplatinum-based chemoradiotherapy
- CI:
-
Confidence interval
- FFPE:
-
Formalin-fixed paraffin-embedded
- Gr:
-
Granzymes
- HNSCC:
-
Head and neck squamous cell carcinoma
- HPV:
-
Human papillomavirus
- IHC:
-
Immunohistochemistry
- OPSCC:
-
Oropharyngeal squamous cell carcinoma
- OS:
-
Overall survival
- PORT:
-
Postoperative radiotherapy
- ROC:
-
Receiver operating characteristic
- RT:
-
Radiotherapy
- TILs:
-
Tumor-infiltrating lymphocytes
- TMA:
-
Tissue microarray
References
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF et al (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35
Marur S, D’Souza G, Westra WH, Forastiere AA (2010) HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 11:781–789
Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E et al (2011) Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29:4294–4301
Hafkamp HC, Manni JJ, Haesevoets A, Voogd AC, Schepers M, Bot FJ et al (2008) Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer 122:2656–2664
Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H et al (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100:261–269
Leemans CR, Braakhuis BJ, Brakenhoff RH (2011) The molecular biology of head and neck cancer. Nat Rev Cancer 11:9–22
Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M (2012) Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol 6(Suppl 1):S104–S120
Igney FH, Krammer PH (2002) Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol 71:907–920
Ward MJ, Thirdborough SM, Mellows T, Riley C, Harris S, Suchak K et al (2014) Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer 110:489–500
Jung AC, Guihard S, Krugell S, Ledrappier S, Brochot A, Dalstein V (2013) CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int J Cancer 132:E26–E36
Nasman A, Romanitan M, Nordfors C, Grün N, Johansson H, Hammarstedt L et al (2012) Tumor infiltrating CD8 + and Foxp3 + lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS ONE 7:e38711. doi:10.1371/journal.pone.0038711
Marzuka A, Huang L, Theodosakis N, Bosenberg M (2015) Melanoma treatments: advances and mechanisms. J Cell Physiol 230:2626–2633
Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ et al (2011) Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17:4550–4557
Lieberman J (2010) Anatomy of a murder: how cytotoxic T cells and NK cells are activated, develop, and eliminate their targets. Immunol Rev 235:5–9
Voskoboinik I, Dunstone MA, Baran K, Whisstock JC, Trapani JA (2010) Perforin: structure, function, and role in human immunopathology. Immunol Rev 235:35–54
Grossman WJ, Revell PA, Lu ZH, Johnson H, Bredemeyer AJ, Ley TJ (2003) The orphan granzymes of humans and mice. Curr Opin Immunol 15:544–552
de Koning PJ, Kummer JA, de Poot SA, Quadir R, Broekhuizen R, McGettrick AF et al (2011) Intracellular serine protease inhibitor SERPINB4 inhibits granzyme M-induced cell death. PLoS ONE 6:e22645. doi:10.1371/journal.pone.0022645
Bird CH, Sutton VR, Sun J, Hirst CE, Novak A, Kumar S (1998) Selective regulation of apoptosis: the cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme B-mediated apoptosis without perturbing the Fas cell death pathway. Mol Cell Biol 18:6387–6398
Wang L, Li Q, Wu L, Liu S, Zhang Y, Yang X et al (2013) Identification of SERPINB1 as a physiological inhibitor of human granzyme H. J Immunol 190:1319–1330
van Diest PJ (2002) No consent should be needed for using leftover body material for scientific purposes. BMJ 325:648–651
van Kempen PM, van Bockel L, Braunius WW, Moelans CB, van Olst M, de Jong R et al (2014) HPV-positive oropharyngeal squamous cell carcinoma is associated with TIMP3 and CADM1 promoter hypermethylation. Cancer Med 3:1185–1196
Camp RL, Charette LA, Rimm DL (2000) Validation of tissue microarray technology in breast carcinoma. Lab Invest 80:1943–1949
Bossard C, Belhadj K, Reyes F, Martin-Garcia N, Berger F, Kummer JA et al (2007) Expression of the granzyme B inhibitor PI9 predicts outcome in nasal NK/T-cell lymphoma: results of a Western series of 48 patients treated with first-line polychemotherapy within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood 109:2183–2189
Badola S, Spurling H, Robinson K, Fedyk ER, Silverman GA, Strayle J et al (2006) Correlation of serpin-protease expression by comparative analysis of real-time PCR profiling data. Genomics 88:173–184
Duray A, Demoulin S, Hubert P, Delvenne P, Saussez S (2010) Immune suppression in head and neck cancers: a review. Clin Dev Immunol 2010:701657. doi:10.1155/2010/701657
Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T et al (2015) Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res 21:632–641
van Kempen PM, Noorlag R, Braunius WW, Stegeman I, Willems SM, Grolman W (2014) Differences in methylation profiles between HPV-positive and HPV-negative oropharynx squamous cell carcinoma: a systematic review. Epigenetics 9:194–203
Krupar R, Robold K, Gaag D, Spanier G, Kreutz M, Renner K et al (2014) Immunologic and metabolic characteristics of HPV-negative and HPV-positive head and neck squamous cell carcinomas are strikingly different. Virchows Arch 465:299–312
Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N et al (2013) PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 73:128–138
Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rödel F et al (2014) Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer 110:501–509
Kong CS, Narasimhan B, Cao H, Kwok S, Erickson JP, Koong A et al (2009) The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 74:553–561
Nordfors C, Grün N, Tertipis N, Ährlund-Richter A, Haeggblom L, Sivars L et al (2013) CD8 + and CD4 + tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur J Cancer 49:2522–2530
Rajjoub S, Basha SR, Einhorn E, Cohen MC, Marvel DM, Sewell DA (2007) Prognostic significance of tumor-infiltrating lymphocytes in oropharyngeal cancer. Ear Nose Throat J 86:506–511
Wansom D, Light E, Thomas D, Worden F, Prince M, Urba S et al (2012) Infiltrating lymphocytes and human papillomavirus-16–associated oropharyngeal cancer. Laryngoscope 122:121–127
Mirghani H, Amen F, Blanchard P, Moreau F, Guigay J, Hartl DM et al (2015) Treatment de-escalation in HPV-positive oropharyngeal carcinoma: ongoing trials, critical issues and perspectives. Int J Cancer 136:1494–1503
Gillison ML, Zhang Q, jordan R, Xiao W, Westra WH, Trotti A et al (2012) Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol 30:2102–2111
Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG et al (2001) The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem 276:33293–33296
Benarafa C, Priebe GP, Remold-O’Donnell E (2007) The neutrophil serine protease inhibitor serpinb1 preserves lung defense functions in Pseudomonas aeruginosa infection. J Exp Med 204:1901–1909
Benarafa C, LeCuyer TE, Baumann M, Stolley JM, Cremona TP, Remold-O’Donnell E (2011) SerpinB1 protects the mature neutrophil reserve in the bone marrow. J Leukoc Biol 90:21–29
Tseng MY, Liu SY, Chen HR, Wu YJ, Chiu CC, Chan PT et al (2009) Serine protease inhibitor (SERPIN) B1 promotes oral cancer cell motility and is over-expressed in invasive oral squamous cell carcinoma. Oral Oncol 45:771–776
Cullen SP, Brunet M, Martin SJ (2010) Granzymes in cancer and immunity. Cell Death Differ 17:616–623
Bovenschen N, Kummer JA (2010) Orphan granzymes find a home. Immunol Rev 235:117–127
van Houdt IS, Oudejans JJ, van den Eertwegh AJ, Baars A, Vos W, Bladergroen BA et al (2005) Expression of the apoptosis inhibitor protease inhibitor 9 predicts clinical outcome in vaccinated patients with stage III and IV melanoma. Clin Cancer Res 11:6400–6407
Bladergroen BA, Strik MC, Bovenschen N, van Berkum O, Scheffer GL, Meijer CJ et al (2001) The granzyme B inhibitor, protease inhibitor 9, is mainly expressed by dendritic cells and at immune-privileged sites. J Immunol 166:3218–3225
Kummer JA, Kamp AM, Tadema TM, Vos W, Meijer CJ, Hack CE (1995) Localization and identification of granzymes A and B-expressing cells in normal human lymphoid tissue and peripheral blood. Clin Exp Immunol 100:164–172
Acknowledgments
The authors would like to thank Domenico Castigliego and Ton Peeters for the help with immunohistochemical staining. S. M. Willems is funded by the Dutch Cancer Society (clinical fellowship: 2011-4964). R. Noorlag is funded by the Dutch Cancer Society (research Grant: 2014-6620).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have a conflict of interest.
Additional information
Rob Noorlag, Justin E. Swartz and Niels Bovenschen have contributed equally to this article.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van Kempen, P.M.W., Noorlag, R., Swartz, J.E. et al. Oropharyngeal squamous cell carcinomas differentially express granzyme inhibitors. Cancer Immunol Immunother 65, 575–585 (2016). https://doi.org/10.1007/s00262-016-1819-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1819-4