Abstract
Purpose
The chromophobe renal cell carcinoma (ChRCC), though associated with a hereditary cancer syndrome, has a good prognosis after tumor removal. The lack of recurrence could be related to the absence of immune system compromise in patients or to an effective functional recovery of immune functions after tumor removal. Thus, we evaluated monocyte-derived dendritic cells (Mo-DCs) in a 34-year-old male who had a ChRCC, before and after tumor removal.
Methods
CD14+ monocytes from the patient’s peripheral blood, 1 week before and 3 months after partial nephrectomy, were differentiated in vitro into immature and mature Mo-DCs. These were harvested, analyzed by flow cytometry and used as stimulators of allogeneic T cells. Supernatants from cultures were collected for cytokine analysis.
Results
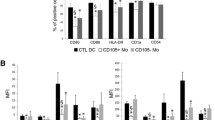
Tumor removal was associated with decreased expression of PD-L1, but also, surprisingly, of CD205, HLA-DR, CD80 and CD86 by Mo-DCs. Also, Mo-DC’s ability to stimulate T cell proliferation increased, along with IL-2Rα expression and IFN-γ production. Simultaneously, the patients’ Mo-DCs ability to induce Foxp3+ T cells decreased after surgery. One-year postoperative follow-up shows no tumor recurrence.
Conclusion
The presence of a ChRCC affected Mo-DCs generated in vitro, which recovered their function after tumor removal. This indicates that the favorable outcome observed after ChRCC resection may be due to the restoration of immunocompetence. Furthermore, since functional alterations described for DCs within tumors may be also found in Mo-DCs, their accurate functional analysis—not restricted to the determination of their surface immunophenotype—may provide an indirect “window” to the tumor microenvironment.




Similar content being viewed by others
Abbreviations
- CFSE:
-
Carboxyfluorescein succinimidyl ester
- ChRCC:
-
Chromophobe renal cell carcinoma
- CT scan:
-
Computed axial tomography
- Foxp3:
-
Forkhead box P3
- FSC:
-
Forward scatter
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- IFN-γ:
-
Interferon gamma
- DCs:
-
Dendritic cells
- IL-2Rα:
-
Alpha chain of interleukin 2 receptor (CD25)
- IL-4:
-
Interleukin 4
- MFI:
-
Media fluorescence intensity
- Mo-DCs:
-
Monocyte-derived dendritic cells
- Mo-iDCs:
-
Monocyte-derived immature dendritic cells
- Mo-mDCs:
-
Monocyte-derived mature dendritic cells
- MRI:
-
Magnetic resonance imaging
- NS Ctrl:
-
Non-stimulated control T cells
- PBMCs:
-
Peripheral blood mononuclear cells
- PD-L1:
-
Programmed cell death ligand 1
- PHA:
-
Phytohaemagglutinin
- SSC:
-
Side scatter
- TNF-α:
-
Tumor necrosis factor alpha
References
Burnet FM (1961) Immunological recognition of self. Science 133(3449):307–311
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331(6024):1565–1570
Steinman RM (2012) Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 30:1–22
Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA (1999) Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11(6):753–761
Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A et al (2013) Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 38(2):336–348
Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M et al (2001) Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 194(6):769–779
Steinman RM, Banchereau J (2007) Taking dendritic cells into medicine. Nature 449(7161):419–426
Waldmann TA (2006) The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 6(8):595–601
Dranoff G (2004) Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer 4(1):11–22
Baleeiro RB, Anselmo LB, Soares FA, Pinto CA, Ramos O, Gross JL et al (2008) High frequency of immature dendritic cells and altered in situ production of interleukin-4 and tumor necrosis factor-alpha in lung cancer. Cancer Immunol Immunother 57(9):1335–1345
Palucka K, Ueno H, Fay J, Banchereau J (2011) Dendritic cells and immunity against cancer. J Intern Med 269(1):64–73
Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP (1997) Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res 3(3):483–490
Enk AH, Jonuleit H, Saloga J, Knop J (1997) Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int J Cancer 73(3):309–316
Della Bella S, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A et al (2003) Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer 89(8):1463–1472
Walker SR, Ogagan PD, DeAlmeida D, Aboka AM, Barksdale EM Jr (2006) Neuroblastoma impairs chemokine-mediated dendritic cell migration in vitro. J Pediatr Surg 41(1):260–265
Ramos RN, Chin LS, Dos Santos AP, Bergami-Santos PC, Laginha F, Barbuto JA (2012) Monocyte-derived dendritic cells from breast cancer patients are biased to induce CD4 + CD25 + Foxp3 + regulatory T cells. J Leukoc Biol 92(3):673–682
Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ (2008) Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3 + CD4 + regulatory T cells. Proc Natl Acad Sci USA 105(27):9331–9336
Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK et al (2009) PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 206(13):3015–3029
Staveley-O’Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S et al (1998) Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc Natl Acad Sci USA 95(3):1178–1183
Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6(4):295–307
Curiel TJ (2008) Regulatory T cells and treatment of cancer. Curr Opin Immunol 20(2):241–246
Crespo J, Sun H, Welling TH, Tian Z, Zou W (2013) T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol 25(2):214–221
Stec R, Grala B, Maczewski M, Bodnar L, Szczylik C (2009) Chromophobe renal cell cancer—review of the literature and potential methods of treating metastatic disease. J Exp Clin Cancer Res 28:134
Singer EA, Bratslavsky G, Linehan WM, Srinivasan R (2010) Targeted therapies for non-clear renal cell carcinoma. Target Oncol 5(2):119–129
Vera-Badillo FE, Conde E, Duran I (2012) Chromophobe renal cell carcinoma: a review of an uncommon entity. Int J Urol 19(10):894–900
Sallusto F, Lanzavecchia A (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179(4):1109–1118
Neron S, Thibault L, Dussault N, Cote G, Ducas E, Pineault N et al (2007) Characterization of mononuclear cells remaining in the leukoreduction system chambers of apheresis instruments after routine platelet collection: a new source of viable human blood cells. Transfusion 47(6):1042–1049
Barbuto JA (2013) Are dysfunctional monocyte-derived dendritic cells in cancer an explanation for cancer vaccine failures? Immunotherapy 5(2):105–107
Shih VF, Davis-Turak J, Macal M, Huang JQ, Ponomarenko J, Kearns JD et al (2012) Control of RelB during dendritic cell activation integrates canonical and noncanonical NF-kappaB pathways. Nat Immunol 13(12):1162–1170
Lutz MB, Schuler G (2002) Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol 23(9):445–449
Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM et al (1995) The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 375(6527):151–155
Mahnke K, Qian Y, Fondel S, Brueck J, Becker C, Enk AH (2005) Targeting of antigens to activated dendritic cells in vivo cures metastatic melanoma in mice. Cancer Res 65(15):7007–7012
Flacher V, Sparber F, Tripp CH, Romani N, Stoitzner P (2009) Targeting of epidermal Langerhans cells with antigenic proteins: attempts to harness their properties for immunotherapy. Cancer Immunol Immunother 58(7):1137–1147
Birkholz K, Schwenkert M, Kellner C, Gross S, Fey G, Schuler-Thurner B et al (2010) Targeting of DEC-205 on human dendritic cells results in efficient MHC class II-restricted antigen presentation. Blood 116(13):2277–2285
Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H et al (2004) In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med 199(6):815–824
Shah W, Yan X, Jing L, Zhou Y, Chen H, Wang Y (2011) A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4(+)FOXP3(+) regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol Immunol 8(1):59–66
Ridgway W, Fasso M, Fathman CG (1998) Following antigen challenge, T cells up-regulate cell surface expression of CD4 in vitro and in vivo. J Immunol 161(2):714–720
Gasocyne RD, Whitney RB, Levy JG (1978) Recovery of immune competence after tumour resection in mice: correlation with loss of suppressor elements. Br J Cancer 37(2):190–198
Lombardi G, Sidhu S, Batchelor R, Lechler R (1994) Anergic T cells as suppressor cells in vitro. Science 264(5165):1587–1589
Sheu BC, Hsu SM, Ho HN, Lin RH, Torng PL, Huang SC (1999) Reversed CD4/CD8 ratios of tumor-infiltrating lymphocytes are correlated with the progression of human cervical carcinoma. Cancer 86(8):1537–1543
Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M et al (2001) Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 61(13):5132–5136
Grabenbauer GG, Lahmer G, Distel L, Niedobitek G (2006) Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res 12(11 Pt 1):3355–3360
Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, Danieli GA et al (1995) Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med 1(12):1279–1283
Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM et al (2007) CD8 + Foxp3 + regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res 13(23):6947–6958
Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Menegaux F et al (2009) Identification of CD8 + CD25 + Foxp3 + suppressive T cells in colorectal cancer tissue. Gut 58(4):520–529
Acknowledgments
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo, FAPESP (2009/54599-5, 2011/05331-0 and 2012/23478-0) and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq. We thank Instituto HOC—Hospital Alemão Oswaldo Cruz, São Paulo—Brazil and Dr. Adilson Kleber Ferreira for the critical reading of this manuscript. This work is dedicated to the patient.
Conflict of interest
The authors declare no financial or other conflict.
Ethical standard
Blood samples and images were collected after written informed consent signed by the patient and the healthy donors, who agreed with the publication of this case report.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Clavijo-Salomon, M.A., Ramos, R.N., Crippa, A. et al. Monocyte-derived dendritic cells reflect the immune functional status of a chromophobe renal cell carcinoma patient: Could it be a general phenomenon?. Cancer Immunol Immunother 64, 161–171 (2015). https://doi.org/10.1007/s00262-014-1625-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1625-9