Abstract
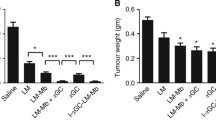
Tumor immunotherapy is currently at the cusp of becoming an important aspect of comprehensive cancer treatment in the clinic. However, the need for improved adjuvants to augment immune responses against tumor antigens is always present. In this paper, we characterize the Listeria monocytogenes-derived actin-nucleating protein, ActA, as a novel adjuvant for use in tumor immunotherapy. ActA is a virulence factor that is expressed on the cell surface of L. monocytogenes and facilitates the production of actin tails that propel Listeria throughout the cytosol of an infected host cell. It is believed that this ActA-dependent cytosolic motility allows Listeria to evade adaptive host cell defenses and facilitates its invasion into a proximal uninfected host cell. However, there is evidence that ActA fused to a tumor antigen and delivered by L. monocytogenes can perform a beneficial function in tumor immunotherapy as an adjuvant. Our investigation of this adjuvant activity demonstrates that ActA, either fused to or administered as a mixture with a tumor antigen, can augment anti-tumor immune responses, break immune tolerance and facilitate tumor eradication, which suggests that ActA is not only an effective adjuvant in tumor immunotherapy but can also be applied in a number of therapeutic settings.






Similar content being viewed by others
References
Coley WB (1991) The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res 262:3–11
Wiemann B, Starnes CO (1994) Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther 64(3):529–564
Morales A, Eidinger D, Bruce AW (1976) Intracavitary Bacillus Calmette–Guerin in the treatment of superficial bladder tumors. J Urol 116(2):180–183
Ratliff TL (1991) Bacillus Calmette–Guerin (BCG): mechanism of action in superficial bladder cancer. Urology 37(Suppl 5):8–11
Guirnalda P, Wood L, Seavey MM, Paterson Y (2009) Bacterial based anti-tumor immunotherapeutic strategies. In: Morrow J, Schmidt C, Davies H, Sheikh N (eds) Vaccinology: principles and practice, Chapter 19. Wiley-Blackwell, NJ
Yuan S, Shi C, Han W, Ling R, Li N, Wang T (2009) Effective anti-tumor responses induced by recombinant Bacillus Calmette–Guerin vaccines based on different tandem repeats of MUC1 and GM-CSF. Eur J Cancer Prev 18(5):416–423
Wallecha A, Carroll KD, Maciag PC, Rivera S, Shahabi V, Paterson Y (2009) Multiple effector mechanisms induced by recombinant Listeria monocytogenes anticancer immunotherapeutics. Adv Appl Microbiol 66:1–27
Singh R, Paterson Y (2006) Listeria monocytogenes as a vector for tumor-associated antigens for cancer immunotherapy. Expert Rev Vaccines 5(4):541–552
Sewell DA, Shahabi V, Gunn GR, Pan Z-K, Dominiecki ME, Paterson Y (2004) Recombinant Listeria vaccines containing PEST sequences are potent immune adjuvants for the tumor-associated antigen HPV-16 E7. Cancer Res 64(24):8821–8825
Peng X, Treml J, Paterson Y (2007) Adjuvant properties of listeriolysin O protein in a DNA vaccination strategy. Cancer Immunol Immunother 56(6):797–806
Tobian AA, Harding CV, Canaday DH (2005) Mycobacterium tuberculosis heat shock fusion protein enhances class I MHC cross-processing and presentation by B lymphocytes. J Immunol 174(9):5209–5214
Schnupf P, Zhou J, Varshavsky A, Portnoy DA (2007) Listeriolysin O secreted by Listeria monocytogenes into the host cell cytosol is degraded by the N-end rule pathway. Infect Immun 75(11):5135–5147
Gunn GR, Zubair A, Peters CH, Pan Z-K, Wu T-C, Paterson Y (2001) Two L. monocytogenes vaccine vectors that express different molecular forms of HPV-16 E7 induce qualitatively different T-cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol 167(11):6471–6479
Kohda C, Kawamura I, Baba H, Nomura T, Ito Y, Kimoto T et al (2002) Dissociated linkage of cytokine-inducing activity and cytotoxicity to different domains of listeriolysin O from Listeria monocytogenes. Infect Immun 70(3):1334–1341
Hamon MA, Batsche E, Regnault B, Tham TN, Seveau S, Muchardt C et al (2007) Histone modifications induced by a family of bacterial toxins. Proc Natl Acad Sci USA 104(33):13467–13472
Krieg AM (2007) Development of TLR9 agonists for cancer therapy. J Clin Invest 117(5):1184–1194
Sewell DA, Douven D, Pan ZK, Rodriguez A, Paterson Y (2004) Regression of HPV-positive tumors treated with a new Listeria monocytogenes vaccine. Arch Otolaryngol Head Neck Surg 130(1):92–97
Souders NC, Sewell DA, Pan ZK, Hussain SF, Rodriguez A, Wallecha A et al (2007) Listeria-based vaccines can overcome tolerance by expanding low avidity CD8 + T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immun 7:2
Domann E, Wehland J, Rohde M, Pistor S, Hartl M, Goebel W et al (1992) A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J 11(5):1981–1990
Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P (1992) L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68(3):521–531
Welch MD, Iwamatsu A, Mitchison TJ (1997) Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature 385(6613):265–269
Yoshikawa Y, Ogawa M, Hain T, Yoshida M, Fukumatsu M, Kim M et al (2009) Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat Cell Biol 11(10):1233–1240
Moors MA, Auerbuch V, Portnoy DA (1999) Stability of the Listeria monocytogenes ActA protein in mammalian cells is regulated by the N-end rule pathway. Cell Microbiol 1(3):249–257
Ledent C, Marcotte A, Dumont JE, Vassart G, Parmentier M (1995) Differentiated carcinomas develop as a consequence of the thyroid-specific expression of a thyroglobulin-human papillomavirus type 16 E7 transgene. Oncogene 10(9):1789–1797
Ji H, Chang EY, Lin KY, Kurman RJ, Pardoll DM, Wu TC (1998) Antigen-specific immunotherapy for murine lung metastatic tumors expressing human papillomavirus type 16 E7 oncoprotein. Int J Cancer 78(1):41–45
Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW et al (1993) Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol 23(9):2242–2249
Schrieber H (2003) Tumor Immunology. In: Paul WE (ed) Fundamental Immunology. Lippincott Williams and Wilkins, Philadelphia, pp 1557–1592
Houghton AN (1994) Cancer antigens: immune recognition of self and altered self. J Exp Med 180(1):1–4
Bruley-Rosset M, Florentin I, Mathe G (1976) In vivo and in vitro macrophage activation by systemic adjuvants. Agents Actions 6(1–3):251–255
Hartmann G, Krieg AM (1999) CpG DNA and LPS induce distinct patterns of activation in human monocytes. Gene Ther 6(5):893–903
Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM (1996) CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA 93(7):2879–2883
D’Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M et al (1992) Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med 176(5):1387–1398
Wolf SF, Sieburth D, Sypek J (1994) Interleukin 12: a key modulator of immune function. Stem Cells 12(2):154–168
Pistor S, Chakraborty T, Walter U, Wehland J (1995) The bacterial actin-nucleator protein ActA of Listeria monocytogenes contains multiple binding sites for host microfilament proteins. Curr Biol 5(5):517–525
Swanson JA (2008) Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol 9(8):639–649
Burkhardt JK, Carrizosa E, Shaffer MH (2008) The actin cytoskeleton in T-cell activation. Annu Rev Immunol 26:233–259
Acknowledgments
This work was supported by grant number CA69632 from the National Institutes of Health. Laurence Wood was supported in part by the NIH/NCI-sponsored program T32 CA09140, Training Program in Immunobiology of Normal and Neoplastic Lymphocytes.
Conflict of interest statement
Yvonne Paterson wishes to disclose that she has a financial interest in Advaxis, a vaccine and therapeutic company that has licensed or has an option to license all patents from the University of Pennsylvania that concern the use of Listeria monocytogenes or listerial products as vaccines.
Author information
Authors and Affiliations
Corresponding author
Additional information
L. M. Wood and Z.-K. Pan contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Wood, L.M., Pan, ZK., Shahabi, V. et al. Listeria-derived ActA is an effective adjuvant for primary and metastatic tumor immunotherapy. Cancer Immunol Immunother 59, 1049–1058 (2010). https://doi.org/10.1007/s00262-010-0830-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-010-0830-4