Abstract
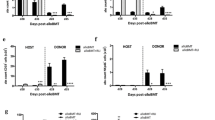
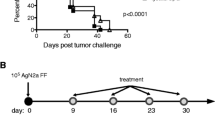
Continuous efforts are dedicated to develop immunotherapeutic approaches to neuroblastoma (NB), a tumor that relapses at high rates following high-dose conventional cytotoxic therapy and autologous bone marrow cell (BMC) reconstitution. This study presents a series of transplant experiments aiming to evaluate the efficacy of allogeneic BMC transplantation. Neuro-2a cells were found to express low levels of class I major histocompatibility complex (MHC) antigens. While radiation and syngeneic bone marrow transplantation (BMT) reduced tumor growth (P < 0.001), allogeneic BMT further impaired subcutaneous development of Neuro-2a cells (P < 0.001). Allogeneic donor-derived T cells displayed direct cytotoxic activity against Neuro-2a in vitro, a mechanism of immune-mediated suppression of tumor growth. The proliferation of lymphocytes from congenic mice bearing subcutaneous tumors was inhibited by tumor lysate, suggesting that a soluble factor suppresses cytotoxic activity of syngeneic lymphocytes. However, the growth of Neuro-2a cells was impaired when implanted into chimeric mice at various times after syngeneic and allogeneic BMT. F1 (donor-host) splenocytes were infused attempting to foster immune reconstitution, however they engrafted transiently and had no effect on tumor growth. Taken together, these data indicate: (1) Neuro-2a cells express MHC antigens and immunogenic tumor associated antigens. (2) Allogeneic BMT is a significantly better platform to develop graft versus tumor (GVT) immunotherapy to NB as compared to syngeneic (autologous) immuno-hematopoietic reconstitution. (3) An effective GVT reaction in tumor bearing mice is primed by MHC disparity and targets tumor associated antigens.





Similar content being viewed by others
References
Ladenstein R, Pötschger U, Hartman O, EBMT Paediatric Working Party et al (2008) 28 years of high-dose therapy and SCT for neuroblastoma in Europe: lessons from more than 4000 procedures. Bone Marrow Transplant 41(Suppl 2):S118–S127
Kanold J, Yakouben K, Tchirkov A et al (2000) Long-term results of CD34(+) cell transplantation in children with neuroblastoma. Med Pediatr Oncol 35:1–7
Burchill SA, Kinsey SE, Picton S et al (2001) Minimal residual disease at the time of peripheral blood stem cell harvest in patients with advanced neuroblastoma. Med Pediatr Oncol 36:213–219
Bowman LC, Grossmann M, Rill D et al (1998) Interleukin-2 gene-modified allogeneic tumor cells for treatment of relapsed neuroblastoma. Hum Gene Ther 9:1303–1311
Lode HN, Xiang R, Dreier T et al (1998) Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood 91:1706–1715
Haight AE, Bowman LC, Ng CY et al (2000) Humoral response to vaccination with interleukin-2-expressing allogeneic neuroblastoma cells after primary therapy. Med Pediatr Oncol 35:712–715
Slavin S, Morecki S, Weiss L, Or R (2002) Donor lymphocyte infusion: the use of alloreactive and tumor-reactive lymphocytes for immunotherapy of malignant and nonmalignant diseases in conjunction with allogeneic stem cell transplantation. J Hematother Stem Cell Res 11:265–276
Kanold J, Paillard C, Tchirkov A et al (2008) Allogeneic or haploidentical HSCT for refractory or relapsed solid tumors in children: toward a neuroblastoma model. Bone Marrow Transplant 42(Suppl 2):S25–S30
Teshima T, Mach N, Hill GR et al (2001) Tumor cell vaccine elicits potent antitumor immunity after allogeneic T-cell-depleted bone marrow transplantation. Cancer Res 61:162–171
Inoue M, Nakano T, Yoneda A et al (2003) Graft-versus-tumor effect in a patient with advanced neuroblastoma who received HLA haplo-identical bone marrow transplantation. Bone Marrow Transplant 32:103–106
Li JM, Waller EK (2004) Donor antigen-presenting cells regulate T-cell expansion and antitumor activity after allogeneic bone marrow transplantation. Biol Blood Marrow Transplant 10:540–551
Hirayama M, Azuma E, Araki M et al (2006) Evidence of graft-versus-tumor effect in refractory metastatic neuroblastoma. Transplantation 82:142–144
Sykulev Y, Joo M, Vturina I et al (1996) Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 4:565–571
Main EK, Lampson LA, Hart MK et al (1985) Human neuroblastoma cell lines are susceptible to lysis by natural killer cells but not by cytotoxic T lymphocytes. J Immunol 135:242–246
Coze C, Aalto-Setala K, Brenner M, Chiang Y (1995) Characteristics and immunomodulatory properties of human neuroblastoma cells after retrovirus-mediated gene transfer of the cytokine genes IL-2 and IFN-gamma. Transgenics 1:585–595
Ponzoni M, Guarnaccia F, Corrias MV, Cornaglia-Ferraris P (1993) Uncoordinate induction and differential regulation of HLA class-I and class-II expression by gamma-interferon in differentiating human neuroblastoma cells. Int J Cancer 55:817–823
Murphy C, Nikodem D, Howcroft K et al (1996) Active repression of major histocompatibility complex class I genes in a human neuroblastoma cell line. J Biol Chem 271:30992–30999
Wolfl M, Jungbluth AA, Garrido F et al (2005) Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol Immunother 54:400–406
Evans A, Main E, Zier K et al (1989) The effects of gamma interferon on the natural killer and tumor cells of children with neuroblastoma: a preliminary report. Cancer 64:1383–1387
Lampson LA, Whelan JP, Fisher CA (1985) HLA-A, B, C and beta 2-microglobulin are expressed weakly by human cells of neuronal origin, but can be induced in neuroblastoma cell lines by interferon. Prog Clin Biol Res 175:379–388
Sugimoto T, Horii Y, Hino T et al (1989) Differential susceptibility of HLA class II antigens induced by gammainterferon in human neuroblastoma cell lines. Cancer Res 49:1824–1828
Hock H, Dorsch M, Kunzendorf U et al (1993) Mechanisms of rejection induced by tumor cell-targeted gene transfer of interleukin 2, interleukin 4, interleukin 7, tumor necrosis factor, or interferon gamma. Proc Natl Acad Sci USA 90:2774–2778
Sadanaga N, Nagoshi M, Lederer JA et al (1999) Local secretion of IFN-gamma induces an antitumor response: comparison between T cells plus IL-2 and IFN-gamma transfected tumor cells. J Immunother 22:315–323
Katsanis E, Orchard PJ, Bausero MA et al (1994) Interleukin-2 gene transfer into murine neuroblastoma decreases tumorigenicity and enhances systemic immunity causing regression of preestablished retroperitoneal tumors. J Immunother 15:81–90
Bauer M, Reaman GH, Hank JA et al (1995) A phase II trial of human recombinant interleukin-2 administered as a 4-day continuous infusion for children with refractory neuroblastoma, non-Hodgkin’s lymphoma, sarcoma, renal cell carcinoma, and malignant melanoma. A Childrens Cancer Group study. Cancer 75:2959–2965
Bowman L, Grossmann M, Rill D et al (1998) IL-2 adenovector-transduced autologous tumor cells induce antitumor immune responses in patients with neuroblastoma. Blood 92:1941–1949
Rousseau RF, Haight AE, Hirschmann-Jax C et al (2003) Local and systemic effects of an allogeneic tumor cell vaccine combining transgenic human lymphotactin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood 101:1718–1726
Pearl-Yafe M, Yolcu ES, Stein J et al (2007) Expression of Fas and Fas-ligand in donor hematopoietic stem and progenitor cells is dissociated from the sensitivity to apoptosis. Exp Hematol 35:1601–1612
Barker SE, Grosse SM, Siapati EK et al (2007) Immunotherapy for neuroblastoma using syngeneic fibroblasts transfected with IL-2 and IL-12. Br J Cancer 97:210–217
Christinck ER, Luscher MA, Barber BH, Williams DB (1991) Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature 352:67–70
Kageyama S, Tsomides TJ, Sykulev Y, Eisen HN (1995) Variations in the number of peptide-MHC class I complexes required to activate cytotoxic T cell responses. J Immunol 154:567–576
Foss FM (2002) Immunologic mechanisms of antitumor activity. Semin Oncol 29:5–11
Saito M, Yu RK, Cheung NK (1985) Ganglioside GD2 specificity of monoclonal antibodies to human neuroblastoma cell. Biochem Biophys Res Commun 127:1–7
Croce M, Meazza R, Orengo AM et al (2008) Immunotherapy of neuroblastoma by an Interleukin-21-secreting cell vaccine involves survivin as antigen. Cancer Immunol Immunother 57:1625–1634
Sugimoto T, Tatsumi E, Kemshead JT et al (1984) Determination of cell surface membrane antigens common to both human neuroblastoma and leukemia-lymphoma cell lines by a panel of 38 monoclonal antibodies. J Natl Cancer Inst 73:51–57
Schonmann SM, Iyer J, Laeng H et al (1986) Production and characterization of monoclonal antibodies against human neuroblastoma. Int J Cancer 37:255–262
Takagi S, Fujikawa K, Imai T et al (1995) Identification of a highly specific surface marker of T-cell acute lymphoblastic leukemia and neuroblastoma as a new member of the transmembrane 4 superfamily. Int J Cancer 61:706–715
Castriconi R, Dondero A, Augugliaro R et al (2004) Identification of 4Ig-B7–H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci USA 101:12640–12645
Morisaki T, Matsumoto K, Onishi H et al (2003) Dendritic cell-based combined immunotherapy with autologous tumor-pulsed dendritic cell vaccine and activated T cells for cancer patients: rationale, current progress, and perspectives. Hum Cell 16:175–182
Redlinger RE Jr, Mailliard RB, Barksdale EM Jr (2004) Neuroblastoma and dendritic cell function. Semin Pediatr Surg 13:61–71
Fujii S (2008) Exploiting dendritic cells and natural killer T cells in immunotherapy against malignancies. Trends Immunol 29:242–249
Fernandez NC, Lozier A, Flament C et al (1999) Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 5:405–411
Shimizu T, Berhanu A, Redlinger RE Jr et al (2001) Watkins S, Lotze MT, Barksdale EM Jr. Interleukin-12 transduced dendritic cells induce regression of established murine neuroblastoma. J Pediatr Surg 36:1285–1292
Castriconi R, Dondero A, Cilli M et al (2007) Human NK cell infusions prolong survival of metastatic human neuroblastoma-bearing NOD/scid mice. Cancer Immunol Immunother 56:1733–1742
Shurin GV, Shurin MR, Bykovskaia S et al (2001) Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res 61:363–369
Redlinger RE Jr, Mailliard RB, Barksdale EM Jr (2003) Advanced neuroblastoma impairs dendritic cell function in adoptive immunotherapy. J Pediatr Surg 38:857–862
Yang L, Carbone DP (2004) Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res 92:13–27
Walker SR, Redlinger RE Jr, Barksdale EM Jr (2005) Neuroblastoma-induced inhibition of dendritic cell IL-12 production via abrogation of CD40 expression. J Pediatr Surg 40:244–250
Shurin GV, Gerein V, Lotze MT, Barksdale EM Jr (1998) Apoptosis induced in T cells by human neuroblastoma cells: role of Fas ligand. Nat Immun 16:263–274
Garrido F, Algarra I (2001) MHC antigens and tumor escape from immune surveillance. Adv Cancer Res 83:117–158
Slavin S, Aker M, Shapira MY et al (2003) Reduced-intensity conditioning for the treatment of malignant and life-threatening non-malignant disorders. Clin Transpl 275–282
Stein J, Dini G, Yaniv I, Pediatric Diseases Working Party of the EBMT (2005) The hope and the reality of reduced intensity transplants in children with malignant diseases. Bone Marrow Transplant 35(Suppl 1):S39–S43
Acknowledgments
This work was supported by grants from the Israel Cancer Research Fund, Israel Ministry of Health, Israel Cancer Research Association, and the Frankel Trust for Experimental Bone Marrow Transplantation. The excellent technical assistance of Mrs. Natalia Binkovsy, Mrs. Ela Zuzovsky, and Mrs. Ana Zemlianski is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ash, S., Gigi, V., Askenasy, N. et al. Graft versus neuroblastoma reaction is efficiently elicited by allogeneic bone marrow transplantation through cytolytic activity in the absence of GVHD. Cancer Immunol Immunother 58, 2073–2084 (2009). https://doi.org/10.1007/s00262-009-0715-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-009-0715-6