Abstract
Introduction
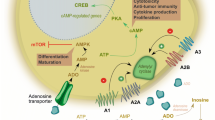
We hypothesize that adenosine and PGE2 could have a complementary immunosuppressive effect that is mediated via common cAMP-PKA signaling.
Materials and methods
To test this hypothesis, the effect of adenosine and PGE2 on the cytotoxic activity and cytokine production of lymphokine activated killer (LAK) cells was investigated.
Results
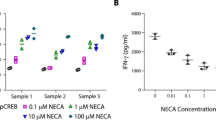
PGE2 and adenosine inhibited LAK cells cytotoxic activity and production of INF-γ, GM-CSF and TNF-α. In combination they showed substantially higher inhibition than each modality used alone. Using agonists and antagonists specific for PGE2 and adenosine receptors we found that cooperation of PGE2 and adenosine in their inhibitory effects are mediated via EP2 and A2A receptors, respectively. LAK cells have 35-fold higher expression of EP2 than A2A. Combined PGE2 and adenosine treatment resulted in augmentation of cAMP production, PKA activity, CREB phosphorylation and inhibition of Akt phosphorylation. Wortmannin and LY294002 enhanced the suppressive effects of adenosine and PGE2. In contrast, Rp-8-Br-cAMPS, an inhibitor of PKA type I blocked their immunosuppressive effects, suggesting that the inhibitory effects of PGE2 and adenosine are mediated via common pathway with activation of cAMP-PKA and inhibition of Akt.
Conclusion
In comparison to other immunosuppressive molecules (TGF-β and IL-10), adenosine and PGE2 are unique in their ability to inhibit the executive function of highly cytotoxic cells. High intratumor levels of adenosine and PGE2 could protect tumor from immune-mediated destruction by inactivation of the tumor infiltrating functionally active immune cells.






Similar content being viewed by others
References
Hata AN, Breyer RM (2004) Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther 103:147–166
Fujino H, Salvi S, Regan JW (2005) Differential regulation of phosphorylation of the cAMP response element-binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Mol Pharmacol 68:251–259
Dannenberg AJ, Subbaramaiah K (2003) Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell 4:431–436
Denkert C, Winzer KJ, Hauptmann S (2004) Prognostic impact of cyclooxygenase-2 in breast cancer. Clin Breast Cancer 4:428–433
Mrena J, Wiksten JP, Thiel A, Kokkola A, Pohjola L, Lundin J, Nordling S, Ristimaki A, Haglund C (2005) Cyclooxygenase-2 is an independent prognostic factor in gastric cancer and its expression is regulated by the messenger RNA stability factor HuR. Clin Cancer Res 11:7362–7368
Eisenthal A (1990) Indomethacin up-regulates the generation of lymphokine-activated killer-cell activity and antibody-dependent cellular cytotoxicity mediated by interleukin-2. Cancer Immunol Immunother 31:342–348
Kundu N, Fulton AM (2002) Selective cyclooxygenase (COX)-1 or COX-2 inhibitors control metastatic disease in a murine model of breast cancer. Cancer Res 62:2343–2346
Kundu N, Walser TC, Ma X, Fulton AM (2005) Cyclooxygenase inhibitors modulate NK activities that control metastatic disease. Cancer Immunol Immunother 54:981–987
Lala PK, Parhar RS (1988) Cure of B16F10 melanoma lung metastasis in mice by chronic indomethacin therapy combined with repeated rounds of interleukin 2: characteristics of killer cells generated in situ. Cancer Res 48:1072–1079
Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, Tiano HF, Morham SG, Smithies O, Langenbach R (2000) Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res 60:4705–4708
Chun KS, Akunda JK, Langenbach R (2007) Cyclooxygenase-2 inhibits UVB-induced apoptosis in mouse skin by activating the prostaglandin E2 receptors, EP2 and EP4. Cancer Res 67:2015–2021
Langenbach R, Loftin CD, Lee C, Tiano H (1999) Cyclooxygenase-deficient mice. A summary of their characteristics and susceptibilities to inflammation and carcinogenesis. Ann N Y Acad Sci 889:52–61
Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM (1996) Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87:803–809
Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T (2001) Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem 276:18563–18569
Ma X, Kundu N, Rifat S, Walser T, Fulton AM (2006) Prostaglandin E receptor EP4 antagonism inhibits breast cancer metastasis. Cancer Res 66:2923–2927
Majima M, Amano H, Hayashi I (2003) Prostanoid receptor signaling relevant to tumor growth and angiogenesis. Trends Pharmacol Sci 24:524–529
Sung YM, He G, Fischer SM (2005) Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res 65:9304–9311
Rozic JG, Chakraborty C, Lala PK (2001) Cyclooxygenase inhibitors retard murine mammary tumor progression by reducing tumor cell migration, invasiveness and angiogenesis. Int J Cancer 93:497–506
Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, Lane TF, Hla T (2004) Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci USA 101:591–596
Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K (2000) Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res 60:1306–1311
Harris SG, Padilla J, Koumas L, Ray D, Phipps RP (2002) Prostaglandins as modulators of immunity. Trends Immunol 23:144–150
Burnstock G (1997) The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology 36:1127–1139
Cronstein BN (1994) Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol 76:5–13
Hasko G, Cronstein BN (2004) Adenosine: an endogenous regulator of innate immunity. Trends Immunol 25:33–39
Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M (2004) Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol 22:657–682
Sitkovsky MV, Ohta A (2005) The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol 26:299–304
Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M (2006) A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA 103:13132–13137
Lokshin A, Raskovalova T, Huang X, Zacharia LC, Jackson EK, Gorelik E (2006) Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res 66:7758–7765
Raskovalova T, Huang X, Sitkovsky M, Zacharia LC, Jackson EK, Gorelik E (2005) Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells. J Immunol 175:4383–4391
Raskovalova T, Lokshin A, Huang X, Jackson EK, Gorelik E (2006) Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-activated NK cells: involvement of protein kinase A isozyme I (PKA I). Immunol Res 36:91–100
Raskovalova T, Lokshin A, Huang X, Su Y, Mandic M, Zarour HM, Jackson EK, Gorelik E (2007) Inhibition of cytokine production and cytotoxic activity of human antimelanoma specific CD8+ and CD4+ T lymphocytes by adenosine-protein kinase A type I signaling. Cancer Res 67:5949–5956
Gunji Y, Vujanovic NL, Hiserodt JC, Herberman RB, Gorelik E (1989) Generation and characterization of purified adherent lymphokine-activated killer cells in mice. J Immunol 142:1748–1754
Ortaldo JR, Bere EW, Hodge D, Young HA (2001) Activating Ly-49 NK receptors: central role in cytokine and chemokine production. J Immunol 166:4994–4999
Gorelik E, Landsittel DP, Marrangoni AM, Modugno F, Velikokhatnaya L, Winans MT, Bigbee WL, Herberman RB, Lokshin AE (2005) Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev 14:981–987
Jackson EK, Zacharia LC, Zhang M, Gillespie DG, Zhu C, Dubey RK (2006) cAMP-adenosine pathway in the proximal tubule. J Pharmacol Exp Ther 317:1219–1229
Fedyk ER, Ripper JM, Brown DM, Phipps RP (1996) A molecular analysis of PGE receptor (EP) expression on normal and transformed B lymphocytes: coexpression of EP1, EP2, EP3beta and EP4. Mol Immunol 33:33–45
Bender AT, Beavo JA (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58:488–520
Skalhegg BS, Tasken K (2000) Specificity in the cAMP/PKA signaling pathway. Differential expression,regulation, and subcellular localization of subunits of PKA. Front Biosci 5:D678–693
Jiang K, Zhong B, Gilvary DL, Corliss BC, Hong-Geller E, Wei S, Djeu JY (2000) Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol 1:419–425
Chin KV, Yang WL, Ravatn R, Kita T, Reitman E, Vettori D, Cvijic ME, Shin M, Iacono L (2002) Reinventing the wheel of cyclic AMP: novel mechanisms of cAMP signaling. Ann N Y Acad Sci 968:49–64
Cho-Chung YS, Nesterova M, Becker KG, Srivastava R, Park YG, Lee YN, Cho YS, Kim MK, Neary C, Cheadle C (2002) Dissecting the circuitry of protein kinase A and cAMP signaling in cancer genesis: antisense, microarray, gene overexpression, and transcription factor decoy. Ann N Y Acad Sci 968:22–36
Torgersen KM, Vaage JT, Levy FO, Hansson V, Rolstad B, Tasken K (1997) Selective activation of cAMP-dependent protein kinase type I inhibits rat natural killer cell cytotoxicity. J Biol Chem 272:5495–5500
Gorelik L, Flavell RA (2001) Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med 7:1118–1122
Kim R, Emi M, Tanabe K, Arihiro K (2006) Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res 66:5527–5536
Desai S, April H, Nwaneshiudu C, Ashby B (2000) Comparison of agonist-induced internalization of the human EP2 and EP4 prostaglandin receptors: role of the carboxyl terminus in EP4 receptor sequestration. Mol Pharmacol 58:1279–1286
Fujino H, Regan JW (2006) EP(4) prostanoid receptor coupling to a pertussis toxin-sensitive inhibitory G protein. Mol Pharmacol 69:5–10
Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hardemark A, Betsholtz C, Herlenius E, Fredholm BB (2001) Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA 98:9407–9412
Gabrilovich D, Pisarev V (2003) Tumor escape from immune response: mechanisms and targets of activity. Curr Drug Targets 4:525–536
Thomas DA, Massague J (2005) TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8:369–380
Koehler H, Kofler D, Hombach A, Abken H (2007) CD28 costimulation overcomes transforming growth factor-beta-mediated repression of proliferation of redirected human CD4+ and CD8+ T cells in an antitumor cell attack. Cancer Res 67:2265–2273
Smeltz RB, Chen J, Shevach EM (2005) Transforming growth factor-beta1 enhances the interferon-gamma-dependent, interleukin-12-independent pathway of T helper 1 cell differentiation. Immunology 114:484–492
Blay J, White TD, Hoskin DW (1997) The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res 57:2602–2605
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, Y., Huang, X., Raskovalova, T. et al. Cooperation of adenosine and prostaglandin E2 (PGE2) in amplification of cAMP–PKA signaling and immunosuppression. Cancer Immunol Immunother 57, 1611–1623 (2008). https://doi.org/10.1007/s00262-008-0494-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0494-5