Abstract
Cancer development is closely associated with immunosuppressive tumor microenvironment (TME) that attenuates antitumor immune responses and promotes tumor cell immunologic escape. The sequential conversion of extracellular ATP into adenosine by two important cell-surface ectonucleosidases CD39 and CD73 play critical roles in reshaping an immunosuppressive TME. The accumulated extracellular adenosine mediates its regulatory functions by binding to one of four adenosine receptors (A1R, A2AR, A2BR and A3R). The A2AR elicits its profound immunosuppressive function via regulating cAMP signaling. The increasing evidence suggests that CD39, CD73 and A2AR could be used as novel therapeutic targets for manipulating the antitumor immunity. In recent years, monoclonal antibodies or small molecule inhibitors targeting the CD39/CD73/A2AR pathway have been investigated in clinical trials as single agents or in combination with anti-PD-1/PD-L1 therapies. In this review, we provide an updated summary about the pathophysiological function of the adenosinergic pathway in cancer development, metastasis and drug resistance. The targeting of one or more components of the adenosinergic pathway for cancer therapy and circumvention of immunotherapy resistance are also discussed. Emerging biomarkers that may be used to guide the selection of CD39/CD73/A2AR-targeting treatment strategies for individual cancer patients is also deliberated.
Similar content being viewed by others
Introduction
Immune homeostasis refers to the tightly regulated balance of immune activation and suppression in our body. While it ensures efficient pathogen recognition and destruction during infection, it prevents excessive and inappropriate self-targeting immune reactions. The accumulating evidences indicate that the majority of cancers are closely associated with failure of this immune homeostasis [1]. Under normal physiological conditions, immune checkpoints play crucial role to protect tissues from damage when the immune system is producing an inflammatory response to fight against pathogenic infection. In cancer cells, the immune checkpoint pathways are highly active and they allow the tumors to evade the antitumor immune response [2]. Immune checkpoint molecules, including inhibitory and stimulatory immune checkpoint molecules, are defined as ligand-receptor pairs that exert inhibitory or stimulatory effects on immune responses, which expresses on immune cells, antigen-presenting cells, tumor cells, or other types of cells, mediating the progress of the adaptive immune system, in particular, T cells and innate immune system. The number of immune checkpoints is increasingly discovered, like PD-1(programmed cell death protein 1), PD-L1(programmed cell death-Ligand 1), LAG3(LymphocyteActivation Gene-3), B7-H3(CD276, Recombinant Cluster Of Differentiation 276), TIM3(T cell immunoglobulin domain and mucin domain-3) [3]. To escape from neoantigen induced antitumor immunity, pathways regulating immune checkpoints are hijacked by tumor cells to induce TIL (Tumor Infiltrating Lymphocyte) exhaustion or suppression. Such as PD-1 and CTLA-4, expressed on activated T cells lead to inhibition of T-cell activation upon binding to their ligands on tumor cells/antigen-presenting cells [4]. The development of immune checkpoint blockade therapy represents a major breakthrough in cancer therapy by unleashing the latent antitumor immune response [5].
In recent years, novel strategies targeting the tumor microenvironment (TME) have emerged as promising therapeutic approaches for cancer treatment [6]. However, while immune checkpoint blockade therapy could produce substantial anticancer effect and durable remission in a small proportion of cancer patients, most patients did not respond due to the presence of immunosuppressive TME [7]. Extracellular adenosine (eADO) activates cell signaling pathways through one of the four known G-protein-coupled adenosine receptors A1, A2A, A2B, and A3. A2A receptors are G-protein-coupled stimulatory pathways that are up-regulated in response to immune cell activation [8]. A2A receptor is a high-affinity receptor expressed on T cells and natural killer T (NKT) cells, monocytes, macrophages, DC (Dendritic cells) and natural killer (NK) cells. A2AR is up-regulated in macrophages in response to NF-κB, STAT1 and PPARγ as well as adenosine signaling, and A2AR activation inhibits the secretion of neutrophil chemokines, thereby reducing the inflammatory response. In effector T cells, increased PKA activity secondary to A2aR signaling has a lots of inhibitory effects, including 1) Inhibiting multiple MAP kinases (ERK1 and JNK); 2) Inhibition of protein kinase C activity, which is important for effector cell activation; 3) Activation of CREB-mediated inhibition of NF-κB and activated T nuclear factor (NF-AT) [9]. Finally, A2AR signal transduction on effectors and regulatory T cells triggers increased expression of other immune checkpoint pathways, including PD-1, CTLA-4(cytotoxic T lymphocyte-associated antigen-4), and LAG-3(lymphocyte activation gene 3) [9]. Thus, the A2AR signal may represent a novel checkpoint pathway. What’s more, the production of adenosine in inflamed tissues combines the regression of inflammation in response to tissue damage with the deep suppression of the immune response by signaling the A2A receptor. However, this combination of wound-healing and immunosuppression is maladaptive in malignancies and is the basic mechanism of cancer immune evasion [10]. To this end, adenosine signaling represents a key metabolic pathway that impairs immunological surveillance [11].
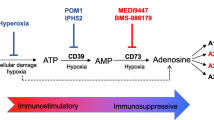
Adenosine is an immunosuppressive metabolite produced at high concentration in TME that contributes to tumor-mediated immune evasion. Under normal conditions, adenosine and ATP are present at low levels in extracellular fluids [12]. The anticancer therapies are known to trigger the release of high levels of ATP to the extracellular compartments, which serves as a Danger-Associated Molecular Pattern (DAMP) to induce both innate and adaptive immune responses [13]. Extracellular ATP is dephosphorylated by ectonucleotidases (CD39 and CD73) to produce adenosine [14]. In contrast to extracellular ATP, adenosine is known to inhibit the activity of the effector immune cells but activate other immunosuppressive regulatory cells [15] (Fig. 1). Therefore, the extent of ATP release to the extracellular compartment and its degradation to adenosine should be limited to restrict the suppressive TME and to facilitate a durable antitumor immunity during cancer immunotherapy [16].
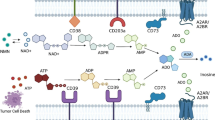
The two ectonucleotidases CD39 and CD73 control the metabolic fate of ATP and adenosine in the extracellular environment. Extracellular ATP is converted into its metabolites ADP and AMP sequentially by CD39, which is then further metabolized to adenosine by CD73. Activated CD39/CD73/A2AR signaling within the TME will suppress the function of antitumor immune cells (T cells, B cells, NK cells, and DCs) but promote the activity of the regulatory immune cells (MDSCs and Tregs), thus giving rise to a immunosuppressive TME. Notes: TME: tumor microenvironment; NK: natural killer; DCs: dendritic cells; MDSC: myeloid-derived suppressor cells; Treg: regulatory T cells; Th17: T helper 17 cells
CD39/CD73/A2AR Signalling within the TME
CD39 and CD73 are highly expressed in various cell types within the TME (including tumor cells, stromal cells, endothelial cells, and the infiltrating immune cells) (Fig. 2) [17]. They are also known to be upregulated in response to the hypoxic tumoral environment. Moreover, both CD39 and CD73 are induced by Tregs (regulatory T cells) in response to adenosine signalling [18, 19], thereby setting up a feedback loop to maintain adenosine production and immunosuppression within the TME. A1R, A2AR and A3R have high affinity for adenosine whereas A2BR has low affinity for adenosine. Upon binding of adenosine to the A2AR or A2BR, cellular adenylyl cyclase activity is increased to raise intracellular cAMP (Cyclic Adenosine monophosphate) level, subsequently inhibiting antitumor immune responses and also activating immune suppressor cells [20, 21].
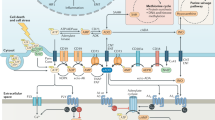
Gene-expression landscape of the three major components (CD39, CD73 and A2AR) in the adenosine signaling pathway in various solid cancer types. The Cancer Genome Altas (TCGA) analysis RNA-sequencing (RNA-seq) data of ENTPD1(A), NT5E (B) and ADORA2A (C), encoding the proteins CD39, CD73, A2AR, respectively, in human cancers. Notes: LUAD: lung adenocarcinoma; LUSC: Lung squamous cell carcinoma; PRAD: Prostate; HNSC: Head and Neck squamous cell; KIRC: Kidney renal clear cell carcinoma; UCEC: Uterinecorps Endometrial carcinoma; PCPG: Pheochromocytoma; LIHC: Liver hepatocellular carcinoma; COAD: Colon adenocarcinoma; READ: Rectum adenocarcinoma; PAAD: Pancreatic adenocarcinoma; BLCA: Bladder Urothelial Carcinoma; CESC: Cervical squamous cell carcinoma; CHOL: Cholangiocarcinoma; ESCA: Esophageal carcinoma; KICH: Kidney renal clear cell carcinoma; KIRP: Kidney renal papillary cell carcinoma; STAD: Stomach adenocarcinoma; THYM: Thyroid carcinoma; THCA: Thyroid carcinoma; BRCA: Breast invasive carcinoma; GBM: Glioblastoma multiforme. N = normal tissue; T = tumor specimen
The CD39 protein (exonucleoside triphosphate diphosphate hydrolase 1; also known as NTPDase 1) has 510 amino acids, which harbors eleven cysteine residues and seven potential N-linked glycosylation sites [22]. There are two transmembrane domains in the CD39 protein. The cytoplasmic domain is relatively short whereas the extracellular domain is large and consists of five highly conserved segments that mediate the nucleotidase activity of the enzyme [23]. CD39 is localized on cell surface and it catalyzes the hydrolysis of extracellular nucleoside tri- and diphosphates to produce the corresponding monophosphates. It is noteworthy that glycosylation of CD39 plays a crucial role to ensure proper protein folding, cell membrane targeting and effective enzymatic activity [24]. The expression of CD39 is induced by a number of inflammatory cytokines, nutrient starvation, oxidative stress, and hypoxia stress via the action of a few transcription factors, including Sp1, Stat3, and the zinc finger protein growth factor independence-1 (GFI1) [25].
The CD73 protein (also known as ecto-5′-nucleotidase) is a glycosyl-phosphatidylinositol-linked cell membrane-bound enzyme found in most tissues [26]. It hydrolyzes the CD39-generated nucleoside monophosphates to the corresponding nucleosides [27]. In particular, CD73 is strongly linked with the generation of adenosine within the TME that stimulates cancer progression by suppressing antitumor immunity and promoting angiogenesis [28].
Extracellular adenosine could be produced by passive diffusion or active transport of intracellular adenosine [29]. On the other hand, it can also be generated by the enzymatic hydrolysis of extracellular ATP. In solid tumors, ATP is released into the extracellular space due to cell necrosis and other secretary mechanisms under the condition of hypoxia, inflammation, nutrient deprivation and cytotoxic drug treatment [30,31,32]. ATP released into the extracellular space is converted to AMP by CD39, and then AMP is further hydrolyzed to adenosine by CD73 [33]. Importantly, both CD39 and CD73 are highly expressed in the cell types within the TME (including tumor cells, immune cells, endothelial cells, and fibroblasts). Moreover, exosomes carrying CD39 and CD73 are constantly released from tumors to enrich the abundance of these ectonucleotidases within the TME. Recently, it has been found that cancer-derived exosomes carries CD39 and CD73 on the surface, and the exosomes from different types of cancer exhibit strong hydrolytic activity of ATP and 5 ‘amp- phosphate, which may be the mechanism that causes adenosine levels to rise in the tumor microenvironment [34,35,36]. Importantly, adenosine is known to suppress the activity of numerous immune cells including phagocytes, dendritic cells (DCs), NK cells (natural killer cells), T cells, B cells, Th17(T helper cell 17), macrophages, upon binding to the A2AR on their cell surface [17, 37]. On the other hand, adenosine can also promote the activity of a few regulatory and suppressive immune cells such as MDSCs (Myeloid-derived suppressor cells) and Tregs to dampen the antitumor immunity [38]. In addition, A2AR has been shown to inhibit macrophage activation by its downstream signaling. Adenosine-A2AR pathway could inhibit T-lymphocyte proliferation, activation, and cytokine production, leading to polarization of immunosuppressive T-regulatory cells [39]. As a result, blockade of A2AR offers a potential next-generation immune checkpoint mechanism for cancer immunotherapy [31].
Recent research has shown that adenosine suppresses immune responses in both CD4+ and CD8+ T cells by regulating the downstream signalling of A2AR [40,41,42,43]. Activation of A2AR by adenosine is known to suppress the proliferation and differentiation of naïve T cells, thus inhibiting Th1 and Th2 differentiation [44]. Moreover, high level of adenosine in the TME also disrupts CD8+ T-cell activation, expansion, and cytokine secretion to inhibit cytotoxic T-cell activity and interferes with NK cells cytolysis activity [37, 45].
B cells are the core component of the adaptive humoral immune system and they work by producing antigen-specific antibodies [46]. However, a growing body of research suggests that B cells could also regulate immune responses through mechanisms beyond antibody production [47]. Human B lymphocytes have been reported to express CD39, CD73, A1R, A2R, and A3R and they can also produce adenosine. The CD39(+)/CD73(+) B cells are capable of producing adenosine, which play critical role in regulating the immune responses of CD4+ and CD8+ T cells [48,49,50]. Human regulatory B cells (Bregs) express high levels of CD39 and they also release IL-10 to suppress T cell–mediated immune responses [51].
In human body, cancer immune surveillance is largely mediated by natural killer (NK) cells. They are effector lymphocytes of the innate immune system that target and kill tumor cells. NK cells are known to be regulated by various metabolic signaling including the purinergic pathway [52]. NK-cell maturation and antitumor immunity are regulated by adenosine signaling through A2AR. Extracellular adenosine interacts with adenosine receptors (predominantly A2AR) expressed on NK cells to mediate suppressive signals [53]. It has been demonstrated that conditional deletion of A2AR could increase the proportion of terminally mature NK cells at homeostasis and also in the TME [54]. Importantly, the specific targeting of A2AR on NK cells has been shown to delay tumor initiation and inhibit tumor growth in animal studies [55]. It is noteworthy that the combination of A2AR antagonists and NK cell–based therapies was shown to promote NK cell-mediated antitumor immunity [56,57,58].
Dendritic cells (DCs) represent the major antigen-presenting cells capable of initiating innate and adaptive immune responses to external pathogens and producing antitumor immunity. Apart from presenting antigens, they can also secrete various cytokines to regulate the immune responses [59, 60]. In DCs, CD39 can affect immunological synapses and intracellular signaling. High concentration of ATP was shown to increase indoleamine-2,3-dioxygenase and thrombospondin 1 levels, which subsequently leads to immunosuppression. The immunosuppressive effect of extracellular ATP and adenosine was related to the decreased secretion of proinflammatory cytokines by DCs [45].
It is commonly believed that regulatory T cells (Tregs) are the prime mediators of immune suppression and they are critical for maintaining peripheral tolerance. They play a key role in protecting against autoimmune diseases and reducing chronic inflammatory conditions, including asthma and inflammatory bowel disease. Besides this important physiological function, Tregs are also known to limit antitumor immunity [61]. To this end, Treg activity can be regulated by the CD39/CD73/A2AR pathway. The activation of adenosine receptor A2AR by extracellular adenosine on Treg cell surface has been shown to stimulate Treg cell proliferation to promote immunosuppression [62].
T helper 17 cells (Th17) are a subset of proinflammatory T helper cells, characterized by their production of interleukin 17 (IL-17). It has been shown that in vitro generated Th17 cells with the cytokines IL-6 and TGF expressed CD39 and CD73, thereby leading to adenosine release and suppression of CD4+ and CD8+ T effector cell functions [63]. On the other hand, the expression level of CD39 and CD73 is decreased in the proinflammatory M1 macrophages, but is increased in the anti-inflammatory M2 macrophages. Therefore, adenosine can indeed promote anti-inflammatory cytokine production but suppress pro-inflammatory cytokine production [64.]. Myeloid-derived suppressor cells (MDSCs) are a heterogeneous group of immature myeloid cells, which suppress T cell response. They are composed of the progenitors of DCs, macrophages, and granulocytes. In the TME, it has been shown that TGF-β and HIF-1α can regulate CD39 and CD73 expression in MDSCs [65].
The mechanism by which the CD39/CD73/adenosine-A2AR suppresses antitumor immunity within TME is depicted in Fig. 1. The immune system plays a vital role in suppressing the development and progression of tumor. Recent research reveals that high levels of immunosuppressive adenosine within the TME contributes substantially to cancer immune evasion. Therefore, the production of high concentration of extracellular adenosine within the TME is mediated by the CD39/CD73/adenosine pathway. The development of novel strategies for immunotherapy by inhibition of this adenosine/A2AR pathway will be discussed in the following sections.
The expression and function of CD39/CD73/A2AR in various Cancer types
Tumor progression and metastasis are regulated by the cross-talk between tumor cells and the TME [66]. CD39 is expressed in infiltrating immune cells as well as on the cancer cells in a range of human cancers, including lung cancer, squamous cell carcinoma of the head and neck, clear cell carcinoma of the kidney, rectal adenocarcinoma, thyroid cancer, breast cancer, and multiforme glioblastoma solid tumors, studies have shown that high expression of CD39 is strongly associated with adverse outcomes [67]. Like CD39, the expression of CD73 in the tumor microenvironment has been studied as a prognostic biomarker for clinical outcomes of a variety of tumor types, including squamous cell carcinoma of the lung, pheochromocytoma, pancreatic cancer, urothelial carcinoma of the bladder, esophageal carcinoma, gastric adenocarcinoma, thyroid carcinoma, and pleomorphic glioblastoma, with metastasis and shorter time to recurrence [68]. In some solid tumors, including lung cancer, pheochromocytoma, hepatocellular carcinoma, bladder urothelial carcinoma, cervical squamous cell carcinoma, and gastric adenocarcinoma, adenosine pathway components are particularly overexpressed, including A2A and A2B. It is expected that these cancers may respond well to drugs targeting the eADO pathway [69]. As showed in Fig. 2, the expression levels of CD39, CD73 and A2AR were higher in several tumor types than their adjacent normal tissues. Moreover, the activation of CD39/CD73/adenosine-A2AR pathway is closely associated with an immunosuppressive TME and poor prognosis of cancer patients [5]. Therefore, CD39 and CD73 are indispensable for the development, differentiation, migration, and invasion of cancer cells [70,71,72]. Importantly, high expression levels of CD39 and CD73 have been associated with immune evasion of cancer cells as they can promote the infiltration of MDSCs and Tregs in tumor tissue [73]. Moreover, the activation of adenosine/A2AR signalling promoted Treg cell proliferation and the secretions of immune-suppressive factors (including TGFβ and IL-10) and upregulated the expression of immune-checkpoint receptors (such as PD-1, CTLA4 and LAG3), which mediated immunosuppression TME in tumor tissue and immune escape of cancer cells [74,75,76].
Given the hypoxic and inflammatory nature of many solid tumors, multiple components of the adenosinergic pathway are upregulated in malignant tissues compared with the respective non-malignant tissues [77]. The CD39 /CD73/A2AR signaling pathway has been shown to be associated with poor cancer prognosis. In addition, CD39 and CD73 are also involved in the formation of new lymphatic vessels around tumors and progression of malignant tumors such as breast carcinoma, multiforme glioblastoma and chronic lymphocytic leukemia [78,79,80]. Importantly, the blockade of adenosine/A2AR pathway resulted in the enhancement of cancer chemotherapy and immunotherapy in numerous cancer types including lung adenocarcinoma, renal clear cell carcinoma, pheochromocytoma and paraganglioma [43, 81,82,83]. Elevated levels of CD39 have also been found in tumors resected for hepatocellular carcinoma, gastric carcinoma, and head and neck squamous cell carcinoma, where higher expression is associated with the likelihood of recurrence after surgery and/or poor overall survival. In addition, FoxP3+ Tregs expressing CD39 were found to be better than FoxP3+ Tregs alone in predicting gastric cancer survival and time to recurrence of HCC [84]. Interestingly, in rectal adenocarcinoma, a combination of CD39 and CD73 expression provided better prognostic value, with CD73hiCD39lo and CD73loCD39hi tumors showing worse and best outcomes, respectively [85]. CD73 may also predict better response to PD-1/PD-L1 targeted therapy, as it is strongly associated with PD-L1 expression in gastrointestinal neuroendocrine tumors [86]. As noted by Antonioli et al., the integration of CD73 and CD39 in prognostic assessment may contribute to enhanced stratification that helps determine the ideal therapeutic strategy in terms of adenosine energy axis’s contribution to cancer progression [87]. And in a smaller cohort of head and neck squamous cell carcinoma, Vogt et al. showed that while hypomethylation of NT5E was associated with worse outcomes, hypomethylation of ADORA2A was associated with longer overall survival [88]. Similarly, et al. found the opposite prognostic value of tumor CD73 and A2A protein expression in two coves of patients with non-small cell lung cancer or lung adenocarcinoma, where CD73 and A2A predicted poorer and better outcomes, respectively, and further studies are needed to better understand the effect of adenosine receptor expression on cancer prognosis [89].
CD39/CD73/A2AR as a novel therapeutic target for combination therapy
Cancer immunotherapy including the PD-1/PD-L1 and CTLA-4 blockade regimens has achieved remarkable anticancer efficacy and long-term survival. However, only a small subset of cancer patients could benefit from the treatment. The fact that a large proportion of cancer patients do not respond suggest the presence of additional immunosuppressive pathway driving the immune evasion by the non-responding tumors [90, 91]. So that the CD39/CD73/A2AR signaling pathway appears to be an attractive target. In solid tumors, abundant ATP is released from the dying cells due to necrosis. CD39 and CD73 are highly expressed in numerous cancer types and also by the infiltrating immune cells. A2AR is expressed in the infiltrating immune cells [92, 93]. Thus, an immunosuppressive environment is reshaped in the TME by the accumulated adenosine to blunt the cancer immune surveillance. In fact, therapeutic targeting of the adenosine signaling has been proposed to enhance the efficacy of other existing cancer immunotherapy [94].
Targeting the CD39/CD73/A2AR pathway
Small-molecule inhibitors and monoclonal antibodies targeting CD39, CD73 and A2AR have been developed for cancer therapy [95]. Generally speaking, monoclonal antibodies (mAb) are macromolecules and they may not penetrate well into solid tumors. In contrast, small molecules could cross physiologic barriers, such as plasma membrane and the blood–brain barrier, more easily. Thus, small-molecule inhibitors could achieve better exposure in the TME [96].
In various tumor models, a CD39-targeting mAb has been shown to inhibit the CD39 enzymatic activity on tumor surface and effectively suppress metastasis [97]. In a lung cancer model, another anti-CD39 mAb was shown to upregulate the expression of CD107a in infiltrating NK cells and promote IFN-γ release to kill cancer cells [98]. ES014 is an anti-CD39/TGF-β bispecific mAb. It was reported to simultaneously inhibit the enzymatic activity of CD39 and neutralize autocrine/paracrine TGF-β, which represent the two major immunosuppressive mechanisms in the TME. Therefore, ES014 could restore anti-tumor immunity by increasing the extracellular levels of the pro-inflammatory ATP, and inhibiting the accumulation of the immunosuppressive adenosine and TGF-β within the TME. Blockade of CD73 by the antagonistic CD73 mAb (3F7) has been shown to significantly delay tumor growth and inhibit metastasis in a 4 T1 breast tumor–bearing mouse model [99]. Moreover, it has been reported that anti-CD73 antibodies could enhance the anticancer effect of both anti-CTLA-4 and anti-PD-1 immunotherapy in multiple tumor-bearing mouse models. These studies also demonstrated that CD73 can inhibit antitumor leukocytes and interfere with adenosine generation to suppress tumor metastasis [100]. In a clinical trial, an anti-CD73 mAb (MEDI9447) with or without durvalumab (PD-L1 mAb) was reported to downregulate CD73 expression on peripheral T cells in 66 pancreatic and colorectal cancer patients, which was associated with an increase in cytotoxic T-cell infiltration [101]. Recently, Pe et al. found that IPH5201 (anti-CD39 mAb) and IPH5301 (anti-CD73 mAb) could efficiently block the hydrolysis of immunogenic ATP into immunosuppressive adenosine by specifically targeting human membrane-associated and soluble forms of CD39 and CD73, respectively. Importantly, IPH5201 and IPH5301 were shown to promote antitumor immunity by stimulating DCs and macrophages and by restoring the activation of T cells isolated from cancer patients [102].
On the other hand, a few small molecule CD39 or CD73 inhibitors are also underway in clinical trials. ES002023 is a CD39 inhibitor which restores antitumor immunity by stabilizing the pro-inflammatory extracellular ATP (eATP) and interfering with synthesis of the immunosuppressive adenosine within the TME (NCT05075564). AB680 is a highly potent, reversible and CD73-selective inhibitor. In preclinical studies, AB680 exhibited favorable pharmacokinetic properties. It is currently being evaluated in phase I clinical trials [103]. PSB-1248937 is another highly potent CD73 inhibitor recently developed but it is not absorbed well by the oral route [104]. There has been extensive search for small molecule CD39/73 inhibitors from natural compounds. Ellagic acid was recently identified as a lead compound for CD39 and CD73 dual inhibitor because of its low cytotoxicity to normal cells [105]. A few allosteric CD73 inhibitors that target the dimer interface have been identified by virtual screening [106]. By exploiting the binding mode of the human protein CD73 with α,β-methylene-ADP, Du et al. designed a series of novel effective small-molecule CD73 inhibitors. Among these CD73 inhibiting drug candidates, OP-5244 was shown to be highly potent and it can be taken orally with high bioavailability [18].
The accumulating preclinical researches demonstrated that the inhibition of A2AR activation can significantly increase antitumor immunity [107]. A2AR inhibitors have been shown to increase antitumor effects by boosting the effector function of cytotoxic lymphocytes and blocking the recruitment and polarization of immunosuppressive immune cells in the TME [108]. A novel A2AR antagonist CPI-444 has been shown to reduce the expression of multiple checkpoint pathways (including PD-1 and LARG-3) on CD8+ effector T cells and CD4+ regulatory T cells. Importantly, A2AR inhibition was found to exhibit the most pronounced effects during CD8+ effector T cell activation, thus remarkably reducing PD-1 and LAG-3 expression at the draining lymph nodes of tumor bearing mice [109]. Mechanistically, it has been demonstrated that the enhancement of IFN-γ production by the adoptively transplanted T lymphocytes contributes to the therapeutic benefit of A2AR antagonism. It is also noteworthy that A2AR antagonism could enhance antitumor immunity regardless of the tumor’s anatomical location and it could provide long-lasting tumor-specific memory [110].
As the adenosine-A2AR pathway is triggered by the binding of adenosine to A2AR to subsequently inhibit T-cell proliferation and function, a few small molecule inhibitors were designed to specifically interfere with the interaction between adenosine and A2AR. The blockage of the binding by ciforadenant and the A2AR inhibitor were reported to restore T-cell signaling, IL-2 and IFN-γ production [57, 111, 112]. AZD4635, a high-affinity oral A2AR antagonist, could reverse T-cell inhibition induced by the treatment with the adenosine analog 5′-n-ethylcarboxylated adenosine in vitro and in vivo [113]. It is currently on phase I clinical trials in patients with a variety of solid tumors [114]. The A2AR antagonist SCH58261 and PBF-509 were shown to block the MSC-mediated suppression of T-cell proliferation almost completely, thereby reactivating the antitumor immune response [115, 116]. We summarizes the various mAbs and small molecule targeting agents of CD39/CD73/A2AR that are currently in clinical trials for cancer therapy in Table 1.
Combination of CD39/CD73/A2AR inhibitors with other therapies
The combination of CD39/CD73/A2AR mAbs or small molecule inhibitors with conventional chemotherapy or other immunotherapies have been investigated in clinical trials on patients with advanced cancer [117, 118]. Additive and even synergistic anticancer effects were achieved in the combination of two distinct antitumor mechanisms. We summarizes the clinical investigations on combination of CD39/CD73/A2AR targeting mAbs or small molecule inhibitors with other cancer treatment modalities in Table 2. Remarkable inhibition of tumor initiation, growth, and metastasis were observed.
Combination of inhibitors targeting two members of the CD39/CD73/A2AR pathway
The adenosine-A2AR pathway consists of different components to convert ATP into the immunosuppressive adenosine. The disruption of individual member of the pathway and their combinations could give rise to different biological effects [119]. Targeted inhibition of A2AR and CD73 was shown to produce synergistic inhibition on tumor growth. The combination of sodium polyoxotungstate (small molecule CD73 inhibitor) and AZD4635 (A2AR antagonist) was found to block the adenosine pathway, thereby activating immune cells, increasing INF-γ production, and reducing the abundance of Treg cells [114]. On the other hand, the combination of IPH5201 (anti-CD39 mAb) and IPH5301 (anti-CD73 mAb) was reported to inhibit the production of adenosine, and subsequently reducing T cell inhibition in a co-culture system of myeloma and stromal cells in vitro [102]. In combination Oleclumab (MEDI9447,anti-CD73 antibody) with AZD4635(A2AR inhibitor), numbers of participants show Dose-limiting Toxicities (DLTs) and numbers of participants show Treatment Emergent Adverse Events (TEAEs) and Treatment Emergent Serious Adverse Events (TESAEs)(NCT03381274).
Combinations of CD39/CD73/A2AR inhibitor with other immunotherapies
The combination of CPI-444 (small molecule A2AR antagonist) and atezolizumab (anti-PD-L1 mAb) was reported to induce more durable anticancer response and more cytotoxic T-cell infiltration in TME than atezolizumab alone [112, 120]. In a recent clinical study, there were substantially more NSCLC patients achieving stable disease when treated with the combination of NIRI178 (A2AR antagonist) and spartalizumab (anti-PD-1 mAb) (14 out of 25) than treatment with apartalizumab alone (7 out of 25) [121]. Importantly, NIR178 with and without spartalizumab was well tolerated in all patients with advanced NSCLC [121]. Similarly, in another clinical trials on patients with advanced metastatic castration-resistant prostate cancer, the combination of AZD4635 (A2AR antagonist) and durvalumab (anti-PD-L1 mAb) was shown to produce more tumor responses (6 out of 37 patients) than treatment with durvalumab alone (2 out of 39 patients) [122]. These clinical data suggests that the inhibitor of CD39/CD73/A2AR pathway can enhance the efficacy of immune checking point inhibitor (ICI) in advanced solid tumorsIn combination PT199 with an anti-PD-1 monoclonal antibody, no loss of inhibition or “hook effect” is observed at a higher concentrations. Hence, PT199 is expected to increase antitumor immune activation, especially in combination with PD-1 pathway inhibition, and thus offer a new treatment option for cancer patients (NCT05431270).
Combinations of CD39/CD73/A2AR inhibitors with other Cancer therapies
The combination of photodynamic therapy and conventional chemotherapy is a promising strategy for destroying cancers that are either under the skin or in the lining of organs reachable by a light source. However, photodynamic therapy is not effective to treating metastatic diseases when tumor cells have already spread [123]. Jin et al. proposed that the combination of anti-CD73 mAb with chemo-photodynamic therapy can synergistically enhance the antimetastatic effects by boosting T cell–mediated antitumor immunity [123]. This approach has been investigated in animal model of metastatic triple-negative breast cancer. While the combination of photodynamic therapy and chemotherapy gave rise to strong antitumor effect and produced immunogenic cell death, the addition of anti-CD73 mAb could assure sufficient immune checkpoint blockade in the tumors by blocking the adenosine pathway [123]. More importantly, this combination strategy was also shown to prevent abscopal tumor metastasis by inducing systemic cytotoxic T cell response via CD73 blockade [123]. However, in a clinical study investigating the combination of IPH5301 (anti-CD73 mAb) with chemotherapy or trastuzumab, dose limiting toxicity of IPH5301 was observed in the combination group. Moreover, similar antitumor response was achieved in the IPH5301-paclitaxel-trastuzumab combination group and the IPH5301 monotherapy group. And the clinical trail of combination dalutrafusp (GS-1423) with mFOLFOX6 regimen was terminated . The decision to discontinue the study was made based on the totality of the clinical, pharmacokinetic, and pharmacodynamic findings (NCT03954704).
It has been proposed that inhibition of adenosine-A2AR pathway could promote the abundance and infiltration of cytotoxic T cells into tumors [124]. Given that the cytokine IL-7 signaling could facilitate the accumulation of tumor-associated CD8+ T cells by hindering adenosine-mediated immunosuppression, the combination of IL-7 modulator and adenosine-A2AR inhibitors have been evaluated for treatment of solid tumors [125]. Newton et al. reported the specific knockdown of A2AR by a lipid nanoparticle-based system to promote the chemotaxis of head and neck cancer memory T cells into the solid tumor [126]. On the other hand, the combination of A2AR antagonists with NK-cell therapy has also been shown to enhance antitumor immunity. DC-based cancer vaccines represent another promising approach for cancer immunotherapy. While efficacy from DC vaccines relies heavily on antitumor T-cell responses [127], cancer cells could utilize the adenosine-A2AR pathway to escape from the antitumor immunity of DC vaccines. So Arabet et al. investigated the potential therapeutic application of combining DC vaccine with inhibitor of the CD39/CD73/A2AR pathway [128]. Apart from promoting angiogenesis and anti-inflammatory activities, CD39 also plays an important role in regulating thrombogenesis to provide adequate blood supply to tumor cells. It was known that tumor cells, endothelial cells, and tumor-infiltrating immune cells express CD39, which suppresses anti-tumor immune responses and promotes tumor growth [129]. Collectively, combination of inhibitor of CD39/CD73/A2AR pathway and cancer immunotherapy is emerged as a novel strategy for treating solid tumors.
Biomarkers of the CD39/CD73/A2AR pathway in Cancer
Recent studies have shown that CD73 is overexpressed in solid tumors such as ovarian, gastric, breast, colorectal cancer [130]. In clinical studies, tumoral CD73 expression was negatively correlated with immune cells infiltration of tumors, worse disease-free survival rate, and poorer overall survival in cancer patients [131]. In prospective clinical trial investigating adenosine pathway inhibitors, inhibition of the CD39/CD73/A2AR pathway was shown to increase immune cell activation, expand T cell repertoire in peripheral blood, and also increase T cell infiltration in tumor biopsy samples [132]. There have been extensive studies investigating pharmacodynamics biomarkers that could predict the clinical responses of adenosine inhibitors. We summarizes the more promising biomarkers used to predict the efficacy of adenosine pathway inhibitors in various cancer types in Table 3.
Since adenosine is metabolized rapidly and its half-life in plasma is only about 10s, it is difficult to directly measure the level of adenosine in patient samples. Therefore, adenosine cannot be used as a biomarker by directly measuring its level in tumor specimens [70]. On the other hand, adenosine-related gene expression profiles were found to correlate well with adenosine levels in tumors. Thus, the profile of adenosine-related gene expression may be used as potential biomarkers to predict treatment response from adenosine-A2AR inhibitors. A recent study revealed that the expression of a group of genes related to myeloid cell biology and inflammation was positively correlated with adenosine levels [96]. A set of 8 genes (including CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL8, PTGS2 and IL-1β) was subsequently coined as the “Adenosine Gene Signature” (AdenoSig) to identify patients likely to respond to treatment with the A2AR antagonist (Ciforadenant) [96, 133]. Meanwhile, Sidders et al. proposed another genomic signature termed the “Adenosine Signaling Score” consisting of 14 genes (PPARG, CYBB, COL3A1, FOXP3, LAG3, APP, CD81, GPI, PTGS2, CASP1, FOS, MAPK1, MAPK3, CREB1), which exhibited good correlation with A2AR signaling in human cancers and could be used to predict immunotherapeutic response [134]. The Adenosine Signaling Score is directly proportional to the concentration of adenosine and it was significantly reduced in A2AR-knockout models. Interestingly, while the AdenoSig and Adenosine Signaling Score only share a single gene in common, they are highly correlated to each other in several solid tumors [133].
On the other hand, decreased adenosine deaminase (ADA) levels in brochoalveolar lavage (BAL) has been used as a diagnostic biomarker for lung cancer. As it is often difficult to obtain sufficient lung tissue from cancer patients for proper diagnosis, ADA levels in BAL could be used as an auxiliary parameter for making malignancy and histopathological diagnoses in conjunction with radiological and clinical findings [139]. It is noteworthy that immunosuppressive functions of CD14high CD163high CD39high macrophages, as well as the secretion of IL-10, were diminished by ADA, thus allowing the measurement of ADA to reflect the status of immunosuppression in the TME [136, 140].
In addition, it has been reported that CD73 expression is upregulated in response to specific oncogenic mutations, including TP53, EGFR and RAS [73]. The expression of CD73 was also correlated well with genes altered by hypoxic and tissue-repair responses, including TGFβ and epithelial-to-mesenchymal transition genes [73]. In various solid tumors, including breast, colorectal, ovarian and pancreatic cancers, cancer-associated fibroblasts (CAFs) constitute the prominent cell population with high expression of CD39 and CD73, which facilitate a feedforward circuit to enforce the CD73 immune checkpoint and maintain an immunosuppressive TME [141]. Furthermore, activation of the EMT was shown to increase CD73 expression and thus eADO receptor signalling, which further enhances the EMT phenotype [72]. Recently, Smyth et al. reported that adenosine signaling could impair the immune effect of peripheral T cells and tumor-infiltrating lymphocytes (TILs) via a A2AR/PKA/mTORC1 signalling pathway [92]. In this study, phosphoflow staining of CREB and S6 proteins was used to assess the influence of adenosine/adenosine receptor on the activation of the PKA and mTOR pathways, respectively Therefore, p-CREB and p-S6 may be used as useful pharmacodynamic and efficacy biomarkers to predict therapeutic response of adenosine-targeting immunotherapies [92]. In summary, various genetic signatures and signalling molecules could be used to select individual cancer patients who may benefit from adenosine-targeting therapy.
Conclusions
The immunosuppressive TME is the major hindrance to successful cancer immunotherapy, which must be overcome in order to achieve robust and durable antitumor response. It has been shown that the purinergic signaling axis contributes to tumor-mediated immunosuppression. The CD39/CD73/adenosine/A2AR signaling is emerging as a promising therapeutic target because adenosine produced by the purine nucleoside in TME can strongly inhibit the immune system. The intratumoral production of adenosine is dependent on the sequential catabolism of ATP by two ectonucleotidases, CD39 (from ATP to AMP) and CD73 (from AMP to adenosine). It is increasingly evidence that CD39/CD73/A2AR pathways play a crucial role in regulating immune responses, both in normal physiology and in pathological states. Importantly, the inhibition of CD73 eliminates a major pathway for adenosine production within the TME and can reverse the immunosuppressive effect mediated by adenosine. Targeting CD39/CD73/A2AR with blocking antibodies or small-molecule inhibitors has exhibited strong antitumor efficacy. In addition, the simultaneous inhibition of CD73 and A2AR was shown to give rise to synergistic effect. Recent findings in the field advocates the development of specific inhibitors targeting CD39/CD73/A2AR to potentiate cancer immunotherapies.
Although both in vitro experiments and animal model studies have confirmed the great potential of targeting CD39/CD73/A2AR pathways for cancer treatment, translating these results into clinical practice will require a deeper understanding of how adenosine regulates the cancer microenvironment. However, one of the deficit in our knowledge is that adenosine promotes cancer growth through its effects on cancer stroma, the direct effects of adenosine on cancer cells are variable. It is also crucial to master a variety of detailed research methods in order to analyze tumor inhibition of adenosine pathways mediated by cancer stroma, such as conditional deletion of adenosine receptors or metabolic enzymes in immune cells or endothelial cells, silencing adenosine receptors or metabolic enzymes in xenograft or allograft prior to inoculation and using a three-dimensional cell culture model that contains cancer cells that constitute their microenvironment. Another factor, the potential use of adenosine drugs in cancer - the intrinsic impact of the adenosine system depends on several factors, including the type of cancer, adenosine receptor subtypes expressed by cancer cells and studies of proliferation, apoptosis or metastasis, such as the fact that a particular tumor may express multiple adenosine receptors, adenosine therapy should take into account these competing proliferative and antiproliferative (or pro-apoptotic and anti-apoptotic) roles of various receptors.
In addition to preclinical studies, clinical studies using adenosine drugs should also rely on a better understanding of specific tumors in humans. Biomarker-based tumor monitoring can guide such adenosine therapy, and these biomarkers may involve various adenosine receptors, metabolic enzymes, and uptake systems, for example, A2B receptor-dependent breast cancer with high expression of A2B receptor can be treated with A2B receptor antagonists. We anticipate that these approaches combined with the analysis of potential polymorphisms in the human adenosine system, will help us to realize the potential of adenosine therapy in the management of cancer patients. With lots of preclinical and clinical studies, the application of inhibitors of the CD39-CD73-A2AR pathway will be broadened and improved. Furthemore,the efficacy of the combination regimen with other immune checkpoint inhibitors has been established and evaluated in preclinical studies. In addition, recent preclinical studies have shown that the benefits of combining CAR T cell therapy with A2AR blocking are quite constructive, investigating such clinical trials and protocols are imminent. Since adenosine production depends on hypoxic conditions and cell renewal, blocking this pathway in combination with therapies that promote hypoxia and cell death within TME should be valuable. These include radiation therapy, which creates hypoxic conditions, and chemotherapy drugs, especially those that increase ATP release (known as “immunogenic chemotherapy”). The diversity of CD39/CD73/A2AR signaling pathway mediated immune mechanisms may indicate its wide application in clinical field.
Availability of data and materials
Not applicable.
Abbreviations
- CD39:
-
Exonucleoside triphosphate diphosphate hydrolase 1
- CD73:
-
Ecto-5′-nucleotidase
- A1R, A2AR, A2BR and A3R:
-
Adenosine A1, A2A, A2B, A3 receptors
- TME:
-
Tumor microenvironment
- DAMP:
-
Danger-Associated Molecular Pattern
- NK:
-
Natural killer
- DCs:
-
Dendritic cells
- MDSC:
-
Myeloid-derived suppressor cells
- Treg:
-
Regulatory T cells
- Th17:
-
T helper 17 cells
- Th1, Th2:
-
T helper 1, 2 cell
- Th Sp1:
-
Transcription factor Sp1
- Stat3:
-
Signal transducer and activator of transcription 3
- GFI1:
-
Growth factor independence-1
- IL-17:
-
Interleukin 17
- EGFR:
-
Epidermal Growth Factor Receptor
- p-CREB:
-
Phospho- CAMP-response element-binding
- CXCR2:
-
C-X-C motif chemokine receptor 2
- TGFβ:
-
Transforming growth factor-β
- LYVE1:
-
Lymphatic Vessel Endothelial receptor-1
- PDPN:
-
Podoplanin
- VEGFC:
-
Vascular endothelial growth factor C
- eATP:
-
Extracellular ATP
- HIF-1α:
-
Hypoxia inducible factor 1 alpha subunit
- CTLA4:
-
Cytotoxic T-lymphocyte associated protein 4
- LAG3:
-
Lymphocyte activation gene 3 protein
- IFN-γ:
-
Interferon γ
- PTGS2:
-
Prostaglandin-endoperoxide synthase 2
- PPARG:
-
Peroxisome proliferative activated receptor, gamma
- CYBB:
-
Cytochrome b-245 beta chain
- COL3A1:
-
Collagen alpha-1(III) chain
- FOXP3:
-
forkhead box P3
- APP:
-
Amyloid Precursor Protein
- GPI:
-
Glucose-6-phosphate isomeras
- CASP1:
-
Caspase 1, apoptosis-related cysteine peptidase
- MAPK:
-
Mitogen-activated protein kinase
- ADA:
-
Adenosine deaminase
- IL-10:
-
Interleukin 10
- CAFs:
-
Cancer-associated fibroblasts
- EMT:
-
Epithelial-MesenchymalTransition
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed cell death-Ligand 1
- B7-H3:
-
CD276, Recombinant Cluster Of Differentiation 276
- TIM3:
-
T cell immunoglobulin domain and mucin domain-3
- TIL:
-
Tumor Infiltrating Lymphocyte
- eADO:
-
Extracellular adenosine.
References
Greten FR, Grivennikov SI. Inflammation and Cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41.
Li B, Chan HL, Chen P. Immune checkpoint inhibitors: basics and challenges. Curr Med Chem. 2019;26(17):3009–25.
Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11.
Zhang Y, Zheng J. Functions of immune checkpoint molecules beyond immune evasion. Adv Exp Med Biol. 2020;1248:201–26.
Baghbani E, Noorolyai S, Shanehbandi D, Mokhtarzadeh A, Aghebati-Maleki L, Shahgoli VK, et al. Regulation of immune responses through CD39 and CD73 in cancer: novel checkpoints. Life Sci. 2021;(282):119826.
Kubli SP, Berger T, Araujo DV, Siu LL, Mak TW. Beyond immune checkpoint blockade: emerging immunological strategies. Nat Rev Drug Discov. 2021;20(12):899–919.
Hammami A, Allard D, Allard B, Stagg J. Targeting the adenosine pathway for cancer immunotherapy. Semin Immunol. 2019;42:101304.
Guo S, Han F, Zhu W. CD39 - a bright target for cancer immunotherapy. Biomed Pharmacother. 2022;151:113066.
Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 2021;14(1):45.
Milne GR, Palmer TM. Anti-inflammatory and immunosuppressive effects of the A2A adenosine receptor. ScientificWorldJournal. 2011;11:320–39.
Wu L, Xie W, Li Y, Ni Q, Timashev P, Lyu M, et al. Biomimetic Nanocarriers guide extracellular ATP homeostasis to remodel energy metabolism for activating innate and adaptive immunity system. Adv Sci (Weinh). 2022;9(17):e2105376.
Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J Immunother Cancer. 2018;6(1):57.
Ahmed A, Tait SWG. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14(12):2994–3006.
Faas MM, Sáez T, de Vos P. Extracellular ATP and adenosine: the Yin and Yang in immune responses? Mol Asp Med. 2017;55:9–19.
Vijayan D, et al. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17(12):709–24.
Boison D, Yegutkin GG. Adenosine metabolism: emerging concepts for Cancer therapy. Cancer Cell. 2019;36(6):582–96.
Li XY, Moesta AK, Xiao C, Nakamura K, Casey M, Zhang H, et al. Targeting CD39 in Cancer reveals an extracellular ATP- and Inflammasome-driven tumor immunity. Cancer Discov. 2019;9(12):1754–73.
Du X, Moore J, Blank BR, Eksterowicz J, Sutimantanapi D, Yuen N, et al. Orally bioavailable small-molecule CD73 inhibitor (OP-5244) reverses immunosuppression through blockade of adenosine production. J Med Chem. 2020;63(18):10433–59.
Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18(12):1332–41.
Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of adenosine receptors: the state of the art. Physiol Rev. 2018;98(3):1591–625.
Zhang T, Yu-Jing L, Ma T. The immunomodulatory function of adenosine in sepsis. Front Immunol. 2022;13:936547.
Moesta AK, Li XY, Smyth MJ. Targeting CD39 in cancer. Nat Rev Immunol. 2020;20(12):739–55.
Paavilainen S, Guidotti G. Interactions between the transmembrane domains of CD39: identification of interacting residues by yeast selection. ScienceOpen Res. 2014;2014. https://doi.org/10.14293/S2199-1006.1.SORLIFE.AEEERM.v1.
Timperi E, Barnaba V. CD39 regulation and functions in T cells. Int J Mol Sci. 2021;22(15):8068.
Zeng J, Ning Z, Wang Y, Xiong H. Implications of CD39 in immune-related diseases. Int Immunopharmacol. 2020;89(Pt A):107055.
Ghalamfarsa G, Kazemi MH, Raoofi Mohseni S, Masjedi A, Hojjat-Farsangi M, Azizi G, et al. CD73 as a potential opportunity for cancer immunotherapy. Expert Opin Ther Targets. 2019;23(2):127–42.
Schneider E, Rissiek A, Winzer R, Puig B, Rissiek B, Haag F, et al. Generation and function of non-cell-bound CD73 in inflammation. Front Immunol. 2019;10:1729.
Chen S, Wainwright DA, Wu JD, Wan Y, Matei DE, Zhang Y, et al. CD73: an emerging checkpoint for cancer immunotherapy. Immunotherapy. 2019;11(11):983–97.
Vultaggio-Poma V, Sarti AC, Di Virgilio F. Extracellular ATP: a feasible target for Cancer therapy. Cells. 2020;9(11):2496.
Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer. 2018;18(10):601–18.
Willingham SB, Hotson AN, Miller RA. Targeting the A2AR in cancer; early lessons from the clinic. Curr Opin Pharmacol. 2020;53:126–33.
Gardani CFF, Cappellari AR, de Souza JB, da Silva BT, Engroff P, Moritz CEJ, et al. Hydrolysis of ATP, ADP, and AMP is increased in blood plasma of prostate cancer patients. Purinergic Signal. 2019;15(1):95–105.
Kepp O, Bezu L, Yamazaki T, Di Virgilio F, Smyth MJ, Kroemer G, et al. ATP and cancer immunosurveillance. EMBO J. 2021;40(13):e108130.
Allard B, Beavis PA, Darcy PK, Stagg J. Immunosuppressive activities of adenosine in cancer. Curr Opin Pharmacol. 2016;29:7–16.
Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev. 2017;276(1):121–44.
Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187(2):676–83.
Sek K, Mølck C, Stewart GD, Kats L, Darcy PK, Beavis PA. Targeting adenosine receptor signaling in Cancer immunotherapy. Int J Mol Sci. 2018;19(12):3837.
Kumar V. Adenosine as an endogenous immunoregulator in cancer pathogenesis: where to go? Purinergic Signal. 2013;9(2):145–65.
Sun C, Wang B, Hao S. Adenosine-A2A receptor pathway in Cancer immunotherapy. Front Immunol. 2022;13:837230.
Shi L, Feng M, Du S, Wei X, Song H, Yixin X, et al. Adenosine generated by regulatory T cells induces CD8+ T cell exhaustion in gastric Cancer through A2aR pathway. Biomed Res Int. 2019;2019:4093214.
Dong LW, Ma ZC, Fu J, Huang BL, Liu FJ, Sun D, et al. Upregulated adenosine 2A receptor accelerates post-infectious irritable bowel syndrome by promoting CD4+ T cells' T helper 17 polarization. World J Gastroenterol. 2022;28(25):2955–67.
Churov A, Zhulai G. Targeting adenosine and regulatory T cells in cancer immunotherapy. Hum Immunol. 2021;82(4):270–8.
Liu G, Zhang Q, Liu G, Li D, Zhang L, Gu Z, et al. Disruption of adenosine 2A receptor improves the anti-tumor function of anti-mesothelin CAR T cells both in vitro and in vivo. Exp Cell Res. 2021;409(1):112886.
Csóka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, et al. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J. 2008;22(10):3491–9.
Canale FP, Ramello MC, Núñez N, Araujo Furlan CL, Bossio SN, Gorosito Serrán M, et al. CD39 expression defines cell exhaustion in tumor-infiltrating CD8+ T cells. Cancer Res. 2018;78(1):115–28.
Sabatino S, JJ JR, Pröbstel AK, Zamvil SS. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci. 2019;20(12):728–45.
Catalán D, Mansilla MA, Ferrier A, Soto L, Oleinika K, Aguillón JC, et al. Immunosuppressive mechanisms of regulatory B cells. Front Immunol. 2021;12:611795.
Saze Z, Schuler PJ, Hong CS, Cheng D, Jackson EK, Whiteside TL. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood. 2013;122(1):9–18.
Shi L, Yang L, Wu Z, Xu W, Song J, Guan W. Adenosine signaling: next checkpoint for gastric cancer immunotherapy? Int Immunopharmacol. 2018;63:58–65.
Passarelli A, Aieta M, Sgambato A, Gridelli C. Targeting Immunometabolism mediated by CD73 pathway in EGFR-mutated non-small cell lung Cancer: a new Hope for overcoming immune resistance. Front Immunol. 2020;11:1479.
Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, van de Veen W. Regulatory B cells. A to Z Allergy. 2021;76(9):2699–715.
Wolf NK, Kissiov DU, Raulet DH. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat Rev Immunol. 2022.
Brauneck F, Seubert E, Wellbrock J, Schulze Zur Wiesch J, Duan Y, Magnus T, et al. Combined blockade of TIGIT and CD39 or A2AR enhances NK-92 cell-mediated cytotoxicity in AML. Int J Mol Sci. 2021;22(23):12919.
Young A, Ngiow SF, Gao Y, Patch AM, Barkauskas DS, Messaoudene M, et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 2018;78(4):1003–16.
Sonigo G, Bozonnat A, Dumont M, Thonnart N, Ram-Wolff C, de Masson A, et al. Involvement of the CD39/CD73/adenosine pathway in T-cell proliferation and NK cell-mediated antibody-dependent cell cytotoxicity in Sézary syndrome. Blood. 2022;139(17):2712–6.
Kamai T, Kijima T, Tsuzuki T, Nukui A, Abe H, Arai K, et al. Increased expression of adenosine 2A receptors in metastatic renal cell carcinoma is associated with poorer response to anti-vascular endothelial growth factor agents and anti-PD-1/anti-CTLA4 antibodies and shorter survival. Cancer Immunol Immunother. 2021;70(7):2009–21.
Leone RD, Lo YC, Powell JD. A2aR antagonists: next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J. 2015;13:265–72.
Cekic C, Day YJ, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014;74(24):7250–9.
Lee YS, Radford KJ. The role of dendritic cells in cancer. Int Rev Cell Mol Biol. 2019;348:123–78.
Sozzani S, Del Prete A, Bosisio D. Dendritic cell recruitment and activation in autoimmunity. J Autoimmun. 2017;85:126–40.
Zhu Y, Zhuang Z, Wu Q, Lin S, Zhao N, Zhang Q, Xie L, Yu S. CD39/CD73/A2a adenosine metabolic pathway: targets for Moxibustion in treating DSS-induced ulcerative colitis. Am J Chin Med 2021;49(3):661–676.
Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol. 2012;(3):190.
Ghiringhelli F, Bruchard M, Chalmin F, Rébé C. Production of adenosine by ectonucleotidases: a key factor in tumor immunoescape. J Biomed Biotechnol. 2012;2012:473712.
Franco R, Lillo A, Rivas-Santisteban R, Reyes-Resina I, Navarro G. Microglial adenosine receptors: from preconditioning to modulating the M1/M2 balance in activated cells. Cells. 2021;10(5):1124.
Chew V, Toh HC, Abastado JP. Immune microenvironment in tumor progression: characteristics and challenges for therapy. J Oncol. 2012;2012:608406.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37.
Shuai C, Xia GQ, Yuan F, Wang S, Lv XW. CD39-mediated ATP-adenosine signalling promotes hepatic stellate cell activation and alcoholic liver disease. Eur J Pharmacol. 2021;905:174198.
de Leve S, Wirsdörfer F, Jendrossek V. The CD73/ado system-a new player in RT induced adverse late effects. Cancers (Basel). 2019;11(10):1578.
Bareche Y, Pommey S, Carneiro M, Buisseret L, Cousineau I, Thebault P, et al. High-dimensional analysis of the adenosine pathway in high-grade serous ovarian cancer. J Immunother Cancer. 2021;9(3):e001965.
Liu J, Shi Y, Liu X, Zhang D, Bai Y, Xu Y, et al. Blocking adenosine/A2AR pathway for Cancer therapy. Zhongguo Fei Ai Za Zhi. 2022;25(7):460–7.
Koszałka P, Pryszlak A, Gołuńska M, Kolasa J, Stasiłojć G, Składanowski AC, et al. Inhibition of CD73 stimulates the migration and invasion of B16F10 melanoma cells in vitro, but results in impaired angiogenesis and reduced melanoma growth in vivo. Oncol Rep. 2014;31(2):819–27.
Iser IC, Vedovatto S, Oliveira FD, Beckenkamp LR, Lenz G, Wink MR. The crossroads of adenosinergic pathway and epithelial-mesenchymal plasticity in cancer. Semin Cancer Biol. 2022;86(Pt 2):202–13.
Allard B, Allard D, Buisseret L, Stagg J. The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol. 2020;17(10):611–29.
Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111(1):251–9.
Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–65.
Bonnereau J, Courau T, Asesio N, Salfati D, Bouhidel F, Corte H, et al. Autologous T cell responses to primary human colorectal cancer spheroids are enhanced by ectonucleotidase inhibition. Gut. 2022;gutjnl-2021-326553. https://doi.org/10.1136/gutjnl-2021-326553. Online ahead of print.
Pang L, Ng KT, Liu J, Yeung WO, Zhu J, Chiu TS, et al. Plasmacytoid dendritic cells recruited by HIF-1α/eADO/ADORA1 signaling induce immunosuppression in hepatocellular carcinoma. Cancer Lett. 2021;522:80–92.
Wang S, Gao S, Zhou D, Qian X, Luan J, Lv X. The role of the CD39-CD73-adenosine pathway in liver disease. J Cell Physiol. 2021;236(2):851–62.
Capone M, Fratangelo F, Giannarelli D, Sorrentino C, Turiello R, Zanotta S, et al. Frequency of circulating CD8+CD73+T cells is associated with survival in nivolumab-treated melanoma patients. J Transl Med. 2020;18(1):121.
de Araújo JB, Kerkhoff VV, de Oliveira Maciel SFV, de Resende E, Silva DT. Targeting the purinergic pathway in breast cancer and its therapeutic applications. Purinergic Signal. 2021;17(2):179–200.
Neo SY, Yang Y, Record J, Ma R, Chen X, Chen Z, et al. CD73 immune checkpoint defines regulatory NK cells within the tumor microenvironment. J Clin Invest. 2020;130(3):1185–98.
Beavis PA, Henderson MA, Giuffrida L, Mills JK, Sek K, Cross RS, et al. Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J Clin Invest. 2017;127(3):929–41.
Giatromanolaki A, Kouroupi M, Pouliliou S, Mitrakas A, Hasan F, Pappa A, et al. Ectonucleotidase CD73 and CD39 expression in non-small cell lung cancer relates to hypoxia and immunosuppressive pathways. Life Sci. 2020;259:118389.
Fu YP, Yi Y, Cai XY, Sun J, Ni XC, He HW, et al. Overexpression of interleukin-35 associates with hepatocellular carcinoma aggressiveness and recurrence after curative resection. Br J Cancer. 2016;114(7):767–76.
Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J, et al. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res. 2015;75(21):4494–503.
Harvey JB, Phan LH, Villarreal OE, Bowser JL. CD73's potential as an immunotherapy target in gastrointestinal cancers. Front Immunol. 2020;11:508.
Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19(6):355–67.
Vogt TJ, Gevensleben H, Dietrich J, Kristiansen G, Bootz F, Landsberg J, et al. Detailed analysis of adenosine A2a receptor (ADORA2A) and CD73 (5′-nucleotidase, ecto, NT5E) methylation and gene expression in head and neck squamous cell carcinoma patients. Oncoimmunology. 2018;7(8):e1452579.
Inoue Y, Yoshimura K, Kurabe N, Kahyo T, Kawase A, Tanahashi M, et al. Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer. Oncotarget. 2017;8(5):8738–51.
Antonia SJ, Vansteenkiste JF, Moon E. Immunotherapy: beyond anti-PD-1 and anti-PD-L1 therapies. Am Soc Clin Oncol Educ Book. 2016;35:e450–8.
Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for Cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561.
Mastelic-Gavillet B, Navarro Rodrigo B, Décombaz L, Wang H, Ercolano G, Ahmed R, et al. Adenosine mediates functional and metabolic suppression of peripheral and tumor-infiltrating CD8+ T cells. J Immunother Cancer. 2019;7(1):257.
Tripathi A, Lin E, Xie W, Flaifel A, Steinharter JA, Stern Gatof EN, et al. Prognostic significance and immune correlates of CD73 expression in renal cell carcinoma. J Immunother Cancer. 2020;8(2):e001467.
Halpin-Veszeleiova K, Hatfield SM. Oxygenation and A2AR blockade to eliminate hypoxia/HIF-1α-adenosinergic immunosuppressive axis and improve cancer immunotherapy. Curr Opin Pharmacol. 2020;53:84–90.
Nocentini A, Capasso C, Supuran CT. Small-molecule CD73 inhibitors for the immunotherapy of cancer: a patent and literature review (2017-present). Expert Opin Ther Pat. 2021;31(10):867–76.
Fong L, Hotson A, Powderly JD, Sznol M, Heist RS, Choueiri TK, et al. Adenosine 2A receptor blockade as an immunotherapy for treatment-refractory renal cell Cancer. Cancer Discov. 2020;10(1):40–53.
Bastid J, Regairaz A, Bonnefoy N, Déjou C, Giustiniani J, Laheurte C, et al. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol Res. 2015;3(3):254–65.
Yan J, Li XY, Roman Aguilera A, Xiao C, Jacoberger-Foissac C, Nowlan B, et al. Control of metastases via myeloid CD39 and NK cell effector function. Cancer Immunol Res. 2020;8(3):356–67.
Qiao Z, Li X, Kang N, Yang Y, Chen C, Wu T, et al. A novel specific anti-CD73 antibody inhibits triple-negative breast Cancer cell motility by regulating autophagy. Int J Mol Sci. 2019;20(5):1057.
Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013;19(20):5626–35.
Hay CM, Sult E, Huang Q, Mulgrew K, Fuhrmann SR, McGlinchey KA, et al. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology. 2016;5(8):e1208875.
Perrot I, Michaud HA, Giraudon-Paoli M, Augier S, Docquier A, Gros L, et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination Cancer therapies. Cell Rep. 2019;27(8):2411–2425.e9.
Lawson KV, Kalisiak J, Lindsey EA, Newcomb ET, Leleti MR, Debien L, et al. Discovery of AB680: a potent and selective inhibitor of CD73. J Med Chem. 2020;63(20):11448–68.
Jeffrey JL, Lawson KV, Powers JP. Targeting metabolism of extracellular nucleotides via inhibition of Ectonucleotidases CD73 and CD39. J Med Chem. 2020;63(22):13444–65.
Wang Y, Wang C, Zhu Y, Zhang Y, Chen B, Wu Y, et al. Discovery of natural product ellagic acid as a potent CD73 and CD39 dual inhibitor. Bioorg Med Chem Lett. 2021;34:127758.
Ripphausen P, Freundlieb M, Brunschweiger A, Zimmermann H, Müller CE, Bajorath J. Virtual screening identifies novel sulfonamide inhibitors of ecto-5′-nucleotidase. J Med Chem. 2012;55(14):6576–81.
Chen L, Li L, Zhou C, Chen X, Cao Y. Adenosine A2A receptor activation reduces brain metastasis via SDF-1/CXCR4 axis and protecting blood-brain barrier. Mol Carcinog. 2020;59(4):390–8.
Sitkovsky M, Lukashev D, Deaglio S, Dwyer K, Robson SC, Ohta A. Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. Br J Pharmacol. 2008;153(Suppl 1):S457–64.
Leone RD, Sun IM, Oh MH, Sun IH, Wen J, Englert J, et al. Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models. Cancer Immunol Immunother. 2018;67(8):1271–84.
Giuffrida L, Sek K, Henderson MA, Lai J, Chen AXY, Meyran D, et al. CRISPR/Cas9 mediated deletion of the adenosine A2A receptor enhances CAR T cell efficacy. Nat Commun. 2021;12(1):3236.
Ma SR, Deng WW, Liu JF, Mao L, Yu GT, Bu LL, et al. Blockade of adenosine A2A receptor enhances CD8+ T cells response and decreases regulatory T cells in head and neck squamous cell carcinoma. Mol Cancer. 2017;16(1):99.
Willingham SB, Ho PY, Hotson A, Hill C, Piccione EC, Hsieh J, et al. A2AR antagonism with CPI-444 induces antitumor responses and augments efficacy to anti-PD-(L)1 and anti-CTLA-4 in preclinical models. Cancer Immunol Res. 2018;6(10):1136–49.
Voronova V, Peskov K, Kosinsky Y, Helmlinger G, Chu L, Borodovsky A, et al. Evaluation of combination strategies for the A2AR inhibitor AZD4635 across tumor microenvironment conditions via a systems pharmacology model. Front Immunol. 2021;12:617316.
Yang R, Elsaadi S, Misund K, Abdollahi P, Vandsemb EN, Moen SH, et al. Conversion of ATP to adenosine by CD39 and CD73 in multiple myeloma can be successfully targeted together with adenosine receptor A2A blockade. J Immunother Cancer. 2020;8(1):e000610.
Yan P, Luo Y, Li X, Li Y, Wang Y, Wu J, et al. A redox-responsive Nanovaccine combined with A2A receptor antagonist for Cancer immunotherapy. Adv Healthc Mater. 2021;10(21):e2101222.
Mediavilla-Varela M, Castro J, Chiappori A, Noyes D, Hernandez DC, Allard B, et al. A novel antagonist of the immune checkpoint protein adenosine A2a receptor restores tumor-infiltrating lymphocyte activity in the context of the tumor microenvironment. Neoplasia. 2017;19(7):530–6.
Goueli SA, Hsiao K. Monitoring and characterizing soluble and membrane-bound ectonucleotidases CD73 and CD39. PLoS One. 2019;14(10):e0220094.
Sitkovsky MV, Hatfield S, Abbott R, Belikoff B, Lukashev D, Ohta A. Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol Res. 2014;2(7):598–605.
Bao X, Xie L. Targeting purinergic pathway to enhance radiotherapy-induced immunogenic cancer cell death. J Exp Clin Cancer Res. 2022;41(1):222.
Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, et al. Adenosine receptor 2A blockade increases the efficacy of anti-PD-1 through enhanced antitumor T-cell responses. Cancer Immunol Res. 2015;3(5):506–17. https://doi.org/10.1158/2326-6066.CIR-14-0211 Epub 2015 Feb 11. PMID: 25672397.
Chiappori AA, Creelan B, Tanvetyanon T, Gray JE, Haura EB, Thapa R, et al. Phase I study of Taminadenant (PBF509/NIR178), an adenosine 2A receptor antagonist, with or without Spartalizumab (PDR001), in patients with advanced non-small cell lung Cancer. Clin Cancer Res. 2022;28(11):2313–20.
Lim EA, Bendell JC, Falchook GS, Bauer TM, Drake CG, Choe JH, et al. Phase 1a/b, Open-label, Multicenter Study of AZD4635 (an Adenosine 2A Receptor Antagonist) as Monotherapy or Combined with Durvalumab, in Patients with Solid Tumors. Clin Cancer Res. 2022;28(22):4871–84.
Jin F, Qi J, Liu D, You Y, Shu G, Du Y, et al. Cancer-cell-biomimetic Upconversion nanoparticles combining chemo-photodynamic therapy and CD73 blockade for metastatic triple-negative breast cancer. J Control Release. 2021;337:90–104.
Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103(35):13132–7.
Koyas A, Tucer S, Kayhan M, Savas AC, Akdemir I, Cekic C. Interleukin-7 protects CD8+ T cells from adenosine-mediated immunosuppression. Sci Signal. 2021;14(674):eabb1269.
Newton HS, Chimote AA, Arnold MJ, Wise-Draper TM, Conforti L. Targeted knockdown of the adenosine A2A receptor by lipid NPs rescues the chemotaxis of head and neck cancer memory T cells. Mol Ther Methods Clin Dev. 2021;21:133–43.
Santos PM, Butterfield LH. Dendritic cell-based Cancer vaccines. J Immunol. 2018;200(2):443–9.
Masjedi A, Ahmadi A, Ghani S, Malakotikhah F, Nabi Afjadi M, Irandoust M, et al. Silencing adenosine A2a receptor enhances dendritic cell-based cancer immunotherapy. Nanomedicine. 2020;29:102240.
Zhang H, Feng L, de Andrade MP, Mao C, Near R, Csizmadia E, et al. Glycoengineered anti-CD39 promotes anticancer responses by depleting suppressive cells and inhibiting angiogenesis in tumor models. J Clin Invest. 2022;132(13):e157431.
Chen Q, Pu N, Yin H, Zhang J, Zhao G, Lou W, et al. CD73 acts as a prognostic biomarker and promotes progression and immune escape in pancreatic cancer. J Cell Mol Med. 2020;24(15):8674–86.
Monteiro I, Missiaglia E, Sciarra A, Santos JV, Bouilly J, Romero P, et al. CD73 expression in normal, hyperplastic, and neoplastic thyroid: a systematic evaluation revealing CD73 overexpression as a feature of papillary carcinomas. Virchows Arch. 2021;479(1):209–14.
Steingold JM, Hatfield SM. Targeting hypoxia-A2A Adenosinergic immunosuppression of antitumor T cells during Cancer immunotherapy. Front Immunol. 2020;11:570041.
Augustin RC, Leone RD, Naing A, Fong L, Bao R, Luke JJ. Next steps for clinical translation of adenosine pathway inhibition in cancer immunotherapy. J Immunother Cancer. 2022;10(2):e004089.
Sidders B, Zhang P, Goodwin K, O'Connor G, Russell DL, Borodovsky A, et al. Adenosine signaling is prognostic for Cancer outcome and has predictive utility for immunotherapeutic response. Clin Cancer Res. 2020;26(9):2176–87.
Sadej R, Skladanowski AC. Dual, enzymatic and non-enzymatic, function of ecto-5′-nucleotidase (eN, CD73) in migration and invasion of A375 melanoma cells. Acta Biochim Pol. 2012;59(4):647–52.
Kutryb-Zajac B, Harasim G, Jedrzejewska A, Krol O, Braczko A, Jablonska P, et al. Macrophage-derived adenosine Deaminase 2 correlates with M2 macrophage phenotype in triple negative breast Cancer. Int J Mol Sci. 2021;22(7):3764.
Allard B, Cousineau I, Allard D, Buisseret L, Pommey S, Chrobak P, et al. Adenosine A2a receptor promotes lymphangiogenesis and lymph node metastasis. Oncoimmunology. 2019;8(8):1601481.
Hamano R, Takahashi HK, Iwagaki H, Kanke T, Liu K, Yoshino T, et al. Stimulation of adenosine A2A receptor inhibits LPS-induced expression of intercellular adhesion molecule 1 and production of TNF-alpha in human peripheral blood mononuclear cells. Shock. 2008;29(2):154–9.
Hocanlı İ, Uzer F, Çil B, Kırhan İ, Günak F. Diagnostic value of adenosine deaminase in bronchoalveolar lavage fluid for patients with lung cancer. Int J Clin Pract. 2021;75(12):e14918.
d'Almeida SM, Kauffenstein G, Roy C, Basset L, Papargyris L, Henrion D, et al. The ecto-ATPDase CD39 is involved in the acquisition of the immunoregulatory phenotype by M-CSF-macrophages and ovarian cancer tumor-associated macrophages: regulatory role of IL-27. Oncoimmunology. 2016;5(7):e1178025.
O'Connor RA, Chauhan V, Mathieson L, Titmarsh H, Koppensteiner L, Young I, et al. T cells drive negative feedback mechanisms in cancer associated fibroblasts, promoting expression of co-inhibitory ligands, CD73 and IL-27 in non-small cell lung cancer. Oncoimmunology. 2021;10(1):1940675.
Acknowledgements
We use biorender (https://biorender.com/) to create our Figures.
Funding
This work was funded by grants from National Natural Science Foundation of China (No.U21A20421,No. 82073882), The Science and Technology Bureau of Foshan (No. FS0AA-KJ218–1301-0008 and No. FS0AA-KJ819–4,901–0082).
Author information
Authors and Affiliations
Contributions
Liwu Fu conceived and designed the manuscrip. Shuanghong Yin and Kenneth K. W. To were involved in drawing the pictures,charting and editing the manuscript. Chenglai Xia participated in writing and modifying the manuscript. All authors review and agreed the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xia, C., Yin, S., To, K.K.W. et al. CD39/CD73/A2AR pathway and cancer immunotherapy. Mol Cancer 22, 44 (2023). https://doi.org/10.1186/s12943-023-01733-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-023-01733-x