Abstract
Objective
This study aims to explore the utility of pretreatment DKI parameters and serum SCC-Ag in evaluating the early therapeutic response of cervical cancer to radiotherapy.
Materials and methods
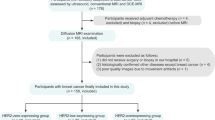
A total of 33 patients diagnosed with cervical cancer, including 31 cases of cervical squamous cell carcinoma and two cases of adenosquamous carcinoma, participated in the study. All patients underwent conventional MRI and DKI scans on a 3T magnetic resonance scanner before radiotherapy and after ten sessions of radiotherapy. The therapeutic response was evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Patients were categorized into a response group (RG), comprising Complete Remission (CR) and Partial Remission (PR), and a non-response group (NRG), comprising Stable Disease (SD) and Progressive Disease (PD). LASSO was employed to select pretreatment DKI parameters, and ROC curves were generated for the selected parameters and serum SCC-Ag.
Results
Significant differences were observed in pretreatment MD, Da, Dr, MK, Ka, Kr, and SCC-Ag between the RG and NRG groups (P < 0.01). However, no significant differences were noted for FA and FAK (P = 0.441&0.928). The two selected parameters (MD and MK) demonstrated area under the curve (AUC), sensitivity, and specificity of 0.810, 0.769, 0.850 and 0.827, 0.846, 0.750, respectively. The combination of MD and MK exhibited an improved AUC of 0.901, sensitivity of 0.692, and specificity of 1.000, with a higher Youden index compared to the individual parameters. Conversely, the AUC, sensitivity, and specificity of the combination of MD, MK, and SCC-Ag were 0.852, 0.615, and 1.000, with a Youden index of 0.615.
Conclusion
Pretreatment MD, MK, and SCC-Ag demonstrate potential clinical utility, with the combined application of MD and MK showing enhanced efficacy in assessing the early therapeutic response of cervical cancer to radiotherapy. The addition of SCC-Ag did not contribute further to the assessment efficacy.
Graphical abstract






Similar content being viewed by others
References
Yang E, Huang S, Ran X, et al. The 5-year overall survival of cervical cancer in stage IIIC-r was little different to stage I and II: a retrospective analysis from a single center. BMC Cancer. 2021;21(1):203.
Ye S, Sun X, Kang B, et al. The kinetic profile and clinical implication of SCC-Ag in squamous cervical cancer patients undergoing radical hysterectomy using the Simoa assay: a prospective observational study. BMC Cancer. 2020;20(1):138.
Chen W, Xiu S, Xie X, et al. Prognostic value of tumor measurement parameters and SCC-Ag changes in patients with locally-advanced cervical cancer. Radiat Oncol. 2022;17(1):6.
Choi KH, Lee SW, Yu M, et al. Significance of elevated SCC-Ag level on tumor recurrence and patient survival in patients with squamous-cell carcinoma of uterine cervix following definitive chemoradiotherapy: a multi-institutional analysis. J Gynecol Oncol. 2019;30(1):e1.
Granata V, Fusco R, Belli A, et al. Diffusion weighted imaging and diffusion kurtosis imaging in abdominal oncological setting: why and when. Infect Agent Cancer. 2022;17(1):25.
Taha HT, Chad JA, Chen JJ. DKI enhances the sensitivity and interpretability of age-related DTI patterns in the white matter of UK biobank participants. Neurobiol Aging. 2022;115:39-49.
Goghari VM, Kusi M, Shakeel MK, et al. Diffusion kurtosis imaging of white matter in bipolar disorder. Psychiatry Res Neuroimaging. 2021 Nov 30;317:111341.
Deng X, Duan Z, Fang S, et al. Advances in The Application and Research of Magnetic Resonance Diffusion Kurtosis Imaging in The Musculoskeletal System. J Magn Reson Imaging. 2023;57(3):670-689.
Peng Q, Tang W, Huang Y, et al. Diffusion kurtosis imaging: correlation analysis of quantitative model parameters with molecular features in advanced lung adenocarcinoma. Chin Med J (Engl). 2020;133(20):2403-2409.
Lo YC, Li TJT, Lin TC, et al. Microstructural Evidence of Neuroinflammation for Psychological Symptoms and Pain in Patients With Fibromyalgia. J Rheumatol. 2022;49(8):942-947.
Hou M, Song K, Ren J, et al. Comparative analysis of the value of amide proton transfer-weighted imaging and diffusion kurtosis imaging in evaluating the histological grade of cervical squamous carcinoma. BMC Cancer. 2022 Jan 20;22(1):87.
Wang M, Perucho JAU, Chan Q, et al. Diffusion Kurtosis Imaging in the Assessment of Cervical Carcinoma. Acad Radiol. 2020;27(5):e94-e101.
Yamada I, Oshima N, Wakana K, et al. Uterine Cervical Carcinoma: Evaluation Using Non-Gaussian Diffusion Kurtosis Imaging and Its Correlation With Histopathological Findings. J Comput Assist Tomogr. 2021;45(1):29-36.
Malek M, Rahmani M, Pourashraf M, et al. Prediction of lymphovascular space invasion in cervical carcinoma using diffusion kurtosis imaging. Cancer Treat Res Commun. 2022;31:100559.
Guo J, Dong C, Wu Z, et al. Diffusion kurtosis imaging assessment of the response to radiotherapy in a VX2 bone tumor model: an animal study. Acta Radiol. 2022;63(2):182-191.
Bhatla N, Aoki D, Sharma DN, et al. Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet. 2021;155 Suppl 1(Suppl 1):28–44.
NCCN Clinical Practice Guidelines in Oncology- Cervical Cancer (2022 Version 1).http://www.nccn.org
Bu X, Zhang J, Tian F, et al. Value of Diffusion-Weighted Magnetic Resonance Imaging Combined with miR-18a Level in Predicting Radiosensitivity of Cervical Cancer. Med Sci Monit. 2018;24:7271-7278.
Cusumano D, Russo L, Gui B, et al. Evaluation of early regression index as response predictor in cervical cancer: A retrospective study on T2 and DWI MR images. Radiother Oncol. 2022;174:30-36.
Zheng X, Chen Y, Xiao Y, et al. Early diagnosis of radio-insensitive human nasopharyngeal carcinoma xenograft models by diffusion kurtosis imaging. Magn Reson Imaging. 2019;55:128-132.
Wang J, Han Y, Li Y, et al. Targeting Tumor Physical Microenvironment for Improved Radiotherapy. Small Methods. 2022;6(11):e2200570.
Tang C, Lu G, Xu J, et al. Diffusion kurtosis imaging and MRI-detected extramural venous invasion in rectal cancer: correlation with clinicopathological prognostic factors. Abdom Radiol (NY). 2023;48(3):844-854.
Cao J, Luo X, Zhou Z, et al. Comparison of diffusion-weighted imaging mono-exponential mode with diffusion kurtosis imaging for predicting pathological grades of clear cell renal cell carcinoma. Eur J Radiol. 2020;130:109195.
Zhang AD, Su XH, Wang YF, et al. Predicting the effects of radiotherapy based on diffusion kurtosis imaging in a xenograft mouse model of esophageal carcinoma. Exp Ther Med. 2021;21(4):327.
Wang W, Lv S, Xun J, et al. Comparison of diffusion kurtosis imaging and dynamic contrast enhanced MRI in prediction of prognostic factors and molecular subtypes in patients with breast cancer. Eur J Radiol. 2022;154:110392.
Kumari S, Advani D, Sharma S, et al. Combinatorial therapy in tumor microenvironment: Where do we stand? Biochim Biophys Acta Rev Cancer. 2021;1876(2):188585.
Yu J, Xu Q, Song JC, et al. The value of diffusion kurtosis magnetic resonance imaging for assessing treatment response of neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol. 2017;27(5):1848-1857.
Granata V, Fusco R, Setola SV, et al. Diffusion kurtosis imaging and conventional diffusion weighted imaging to assess electrochemotherapy response in locally advanced pancreatic cancer. Radiol Oncol. 2019;53(1):15-24.
Meng N, Wang X, Sun J, et al. Application of the amide proton transfer-weighted imaging and diffusion kurtosis imaging in the study of cervical cancer. Eur Radiol. 2020;30(10):5758-5767.
Li X, Yang L, Wang Q, et al. Soft tissue sarcomas: IVIM and DKI correlate with the expression of HIF-1α on direct comparison of MRI and pathological slices. Eur Radiol. 2021;31(7):4669-4679.
Tang C, Qin Y, Hu Q, et al. Diagnostic value of multi-model high-resolution diffusion-weighted MR imaging in breast lesions: Based on simultaneous multi-slice readout-segmented echo-planar imaging. Eur J Radiol. 2022;154:110439.
Cao L, Chen J, Duan T, et al. Diffusion kurtosis imaging (DKI) of hepatocellular carcinoma: correlation with microvascular invasion and histologic grade. Quant Imaging Med Surg. 2019;9(4):590-602.
Yuan SJ, Qiao TK, Qiang JW. Diffusion-weighted imaging and diffusion kurtosis imaging for early evaluation of the response to docetaxel in rat epithelial ovarian cancer. J Transl Med. 2018;16(1):340.
Li Q, Cao B, Tan Q, et al. Prediction of muscle invasion of bladder cancer: A comparison between DKI and conventional DWI.
Guo J, Sun W, Dong C, et al. Intravoxel incoherent motion imaging combined with diffusion kurtosis imaging to assess the response to radiotherapy in a rabbit VX2 malignant bone tumor model. Cancer Imaging. 2022;22(1):47.
Markovina S, Wang S, Henke LE, et al. Serum squamous cell carcinoma antigen as an early indicator of response during therapy of cervical cancer. Br J Cancer. 2018;118(1):72-78.
Benito V, Lubrano A, Pérez-Regadera JF, et al. Postreatment squamous cell carcinoma antigen as a survival prognostic factor in patients with locally advanced cervical cancer. A Spanish multicenter study. The SEGO Spain-GOG group. Gynecol Oncol. 2021;162(2):407–412.
Zhang M, Xin L, Cheng B, et al. Clinical Value Analysis of Serum TK1, SCC-Ag, and MUC-1 in the Diagnosis and Prognosis Evaluation of Cervical Cancer. Altern Ther Health Med. 2023:AT9130.
Zhou Z, Li W, Zhang F, et al. The value of squamous cell carcinoma antigen (SCCa) to determine the lymph nodal metastasis in cervical cancer: A meta-analysis and literature review. PLoS One. 2017;12(12):e0186165.
Fu J, Wang W, Wang Y, et al. The role of squamous cell carcinoma antigen (SCC Ag) in outcome prediction after concurrent chemoradiotherapy and treatment decisions for patients with cervical cancer. Radiat Oncol. 2019;14(1):146.
Yoo JG, Kim SI, Yeo SG, et al. Usefulness of Short-Term Imaging and Squamous Cell Carcinoma Antigen to Early Predict Response to Concurrent Chemoradiotherapy in Patients With Cervical Cancer. Cancer Control. 2022;29:10732748221074530.
Ran C, Sun J, Qu Y, et al. Clinical value of MRI, serum SCCA, and CA125 levels in the diagnosis of lymph node metastasis and para-uterine infiltration in cervical cancer. World J Surg Oncol. 2021;19(1):343.
Funding
Funding was provided by Natural Science Foundation of Fujian Province (2021J01426) to Xiang Zheng.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, X., Shen, F., Chen, W. et al. Integrated pretreatment diffusion kurtosis imaging and serum squamous cell carcinoma antigen levels: a biomarker strategy for early assessment of radiotherapy outcomes in cervical cancer. Abdom Radiol (2024). https://doi.org/10.1007/s00261-024-04270-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00261-024-04270-3