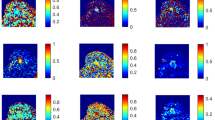
Abstract
Objectives
To determine the value of conventional DWI, continuous-time random walk (CTRW), fractional order calculus (FROC), and stretched exponential model (SEM) in discriminating human epidermal growth factor receptor 2 (HER2) status of breast cancer (BC).
Methods
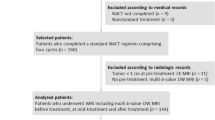
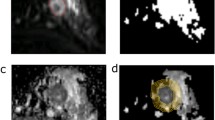
This prospective study included 158 women who underwent DWI, CTRW, FROC, and SEM and were pathologically categorized into the HER2-zero-expressing group (n = 10), HER2-low-expressing group (n = 86), and HER2-overexpressing group (n = 62). Nine diffusion parameters, namely ADC, αCTRW, βCTRW, DCTRW, βFROC, DFROC, μFROC, αSEM, and DDCSEM of the primary tumor, were derived from four diffusion models. These diffusion metrics and clinicopathologic features were compared between groups. Logistic regression was used to determine the optimal diffusion metrics and clinicopathologic variables for classifying the HER2-expressing statuses. Receiver operating characteristic (ROC) curves were used to evaluate their discriminative ability.
Results
The estrogen receptor (ER) status, progesterone receptor (PR) status, and tumor size differed between HER2-low-expressing and HER2-overexpressing groups (p < 0.001 to p = 0.009). The αCTRW, DCTRW, βFROC, DFROC, μFROC, αSEM, and DDCSEM were significantly lower in HER2-low-expressing BCs than those in HER2-overexpressing BCs (p < 0.001 to p = 0.01). Further multivariable logistic regression analysis showed that the αCTRW was the single best discriminative metric, with an area under the curve (AUC) being higher than that of ADC (0.802 vs. 0.610, p < 0.05); the addition of ER status, PR status, and tumor size to the αCTRW improved the AUC to 0.877.
Conclusions
The αCTRW could help discriminate the HER2-low-expressing and HER2-overexpressing BCs.
Clinical relevance statement
Human epidermal growth factor receptor 2 (HER2)-low-expressing breast cancer (BC) might also benefit from the HER2-targeted therapy. Prediction of HER2-low-expressing BC or HER2-overexpressing BC is crucial for appropriate management. Advanced continuous-time random walk diffusion MRI offers a solution to this clinical issue.
Key Points
• Human epidermal receptor 2 (HER2)-low-expressing BC had lower αCTRW, DCTRW, βFROC, DFROC, μFROC, αSEM, and DDCSEM values compared with HER2-overexpressing breast cancer.
• The αCTRW was the single best diffusion metric (AUC = 0.802) for discrimination between the HER2-low-expressing and HER2-overexpressing breast cancers.
• The addition of αCTRW to the clinicopathologic features (estrogen receptor status, progesterone receptor status, and tumor size) further improved the discriminative ability.






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- BC:
-
Breast cancer
- CI:
-
Confidence interval
- CTRW:
-
Continuous-time random walk
- DCE:
-
Dynamic contrast-enhanced
- DTI:
-
Diffusion tensor imaging
- DWI:
-
Diffusion-weighted imaging
- ER:
-
Estrogen receptor
- FISH:
-
Fluorescence in situ hybridization
- FOV:
-
Field of view
- FROC:
-
Fractional order calculus
- HER2:
-
Human epidermal growth factor receptor 2
- ICC:
-
Inter-class correlation coefficient
- IHC:
-
Immunohistochemistry
- MRI:
-
Magnetic resonance imaging
- PR:
-
Progesterone receptor
- QIBA:
-
Quantitative Imaging Biomarkers Alliance
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- SE-EPI:
-
Spin-echo echo-planar imaging
- SEM:
-
Stretched exponential model
- T1WI:
-
T1-weighted imaging
- T2WI:
-
T2-weighted imaging
- TE:
-
Echo time
- TR:
-
Repetition time
- VOI:
-
Volume of interest
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Harbeck N, Penault-Llorca F, Cortes J et al (2019) Breast cancer. Nat Rev Dis Primers 5:66. https://doi.org/10.1038/s41572-019-0111-2
Horvat JV, Bernard-Davila B, Helbich TH et al (2019) Diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) mapping as a quantitative imaging biomarker for prediction of immunohistochemical receptor status, proliferation rate, and molecular subtypes of breast cancer. J Magn Reson Imaging 50:836–846. https://doi.org/10.1002/jmri.26697
Gingras I, Gebhart G, de Azambuja E, Piccart-Gebhart M (2017) HER2-positive breast cancer is lost in translation: time for patient-centered research. Nat Rev Clin Oncol 14:669–681. https://doi.org/10.1038/nrclinonc.2017.96
Modi S, Saura C, Yamashita T et al (2020) Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 382:610–621. https://doi.org/10.1056/NEJMoa1914510
Wolff AC, Hammond ME, Hicks DG et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013. https://doi.org/10.1200/JCO.2013.50.9984
Tarantino P, Hamilton E, Tolaney SM et al (2020) HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol 38:1951–1962. https://doi.org/10.1200/JCO.19.02488
Denkert C, Seither F, Schneeweiss A et al (2021) Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol 22:1151–1161. https://doi.org/10.1016/S1470-2045(21)00301-6
Modi S, Park H, Murthy RK et al (2020) Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol 38:1887–1896. https://doi.org/10.1200/JCO.19.02318
Eiger D, Agostinetto E, Saúde-Conde R, de Azambuja E (2021) The exciting new field of HER2-low breast cancer treatment. Cancers (Basel) 13:1015. https://doi.org/10.3390/cancers13051015
Modi S, Jacot W, Yamashita T et al (2022) Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387:9–20. https://doi.org/10.1056/NEJMoa2203690
Jordan NV, Bardia A, Wittner BS et al (2016) HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 537:102–106. https://doi.org/10.1038/nature19328
Onaygil C, Kaya H, Ugurlu MU, Aribal E (2017) Diagnostic performance of diffusion tensor imaging parameters in breast cancer and correlation with the prognostic factors. J Magn Reson Imaging 45:660–672. https://doi.org/10.1002/jmri.25481
Meng N, Wang X, Sun J et al (2021) A comparative study of the value of amide proton transfer-weighted imaging and diffusion kurtosis imaging in the diagnosis and evaluation of breast cancer. Eur Radiol 31:1707–1717. https://doi.org/10.1007/s00330-020-07169-x
Andreassen MMS, Rodríguez-Soto AE, Conlin CC et al (2021) Discrimination of breast cancer from healthy breast tissue using a three-component diffusion-weighted MRI model. Clin Cancer Res 27:1094–1104. https://doi.org/10.1158/1078-0432.CCR-20-2017
Vidić I, Egnell L, Jerome NP et al (2018) Support vector machine for breast cancer classification using diffusion-weighted MRI histogram features: preliminary study. J Magn Reson Imaging 47:1205–1216. https://doi.org/10.1002/jmri.25873
Mao C, Jiang W, Huang J et al (2022) Quantitative parameters of diffusion spectrum imaging: HER2 status prediction in patients with breast cancer. Front Oncol 12:817070. https://doi.org/10.3389/fonc.2022.817070
Gao A, Zhang H, Yan X et al (2022) Whole-tumor histogram analysis of multiple diffusion metrics for glioma genotyping. Radiology 302:652–661. https://doi.org/10.1148/radiol.210820
Mao J, Zeng W, Zhang Q et al (2020) Differentiation between high-grade gliomas and solitary brain metastases: a comparison of five diffusion-weighted MRI models. BMC Med Imaging 20:124. https://doi.org/10.1186/s12880-020-00524-w
Vidić I, Egnell L, Jerome NP et al (2020) Modeling the diffusion-weighted imaging signal for breast lesions in the b = 200 to 3000 s/mm2 range: quality of fit and classification accuracy for different representations. Magn Reson Med 84:1011–1023. https://doi.org/10.1002/mrm.28161
You C, Li J, Zhi W et al (2019) The volumetric-tumour histogram-based analysis of intravoxel incoherent motion and non-Gaussian diffusion MRI: association with prognostic factors in HER2-positive breast cancer. J Transl Med 17:182. https://doi.org/10.1186/s12967-019-1911-6
Karaman MM, Zhang J, Xie KL, Zhu W, Zhou XJ (2021) Quartile histogram assessment of glioma malignancy using high b-value diffusion MRI with a continuous-time random-walk model. NMR Biomed 34:e4485. https://doi.org/10.1002/nbm.4485
Zhong Z, Merkitch D, Karaman MM et al (2019) High-spatial-resolution diffusion MRI in Parkinson disease: lateral asymmetry of the substantia nigra. Radiology 291:149–157. https://doi.org/10.1148/radiol.2019181042
Sui Y, Wang H, Liu G et al (2015) Differentiation of low- and high-grade pediatric brain tumors with high b-value diffusion-weighted MR imaging and a fractional order calculus model. Radiology 277:489–496. https://doi.org/10.1148/radiol.2015142156
Bai Y, Lin Y, Tian J et al (2016) Grading of gliomas by using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging and diffusion kurtosis MR imaging. Radiology 278:496–504. https://doi.org/10.1148/radiol.2015142173
Bickelhaupt S, Steudle F, Paech D et al (2017) On a fractional order calculus model in diffusion weighted breast imaging to differentiate between malignant and benign breast lesions detected on X-ray screening mammography. PLoS One 12:e0176077. https://doi.org/10.1371/journal.pone.0176077
Suo S, Yin Y, Geng X et al (2021) Diffusion-weighted MRI for predicting pathologic response to neoadjuvant chemotherapy in breast cancer: evaluation with mono-, bi-, and stretched-exponential models. J Transl Med 19:236. https://doi.org/10.1186/s12967-021-02886-3
Suo S, Cheng F, Cao M et al (2017) Multiparametric diffusion-weighted imaging in breast lesions: association with pathologic diagnosis and prognostic factors. J Magn Reson Imaging 46:740–750. https://doi.org/10.1002/jmri.25612
Jin YN, Zhang Y, Cheng JL, Zheng DD, Hu Y (2019) Monoexponential, Biexponential, and stretched-exponential models using diffusion-weighted imaging: a quantitative differentiation of breast lesions at 3.0T. J Magn Reson Imaging 50:1461–1467. https://doi.org/10.1002/jmri.26729
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505. https://doi.org/10.1148/radiology.168.2.3393671
Rahbar H, Partridge SC, Demartini WB et al (2012) In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters. Radiology 263:374–382. https://doi.org/10.1148/radiol.12111368
Hammond ME, Hayes DF, Dowsett M et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795. https://doi.org/10.1200/JCO.2009.25.6529
Dowsett M, Nielsen TO, A’Hern J et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 103:1656–1664. https://doi.org/10.1093/jnci/djr393
Fehrenbacher L, Cecchini RS, Geyer CE Jr et al (2020) NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol 38:444–453. https://doi.org/10.1200/JCO.19.01455
Yuen S, Monzawa S, Yanai S et al (2020) The association between MRI findings and breast cancer subtypes: focused on the combination patterns on diffusion-weighted and T2-weighted images. Breast Cancer 27:1029–1037. https://doi.org/10.1007/s12282-020-01105-z
Jiang Z, Song L, Lu H, Yin J (2019) The potential use of DCE-MRI texture analysis to predict HER2 2+ status. Front Oncol 9:242. https://doi.org/10.3389/fonc.2019.00242
Tang L, Zhou XJ (2019) Diffusion MRI of cancer: from low to high b-values. J Magn Reson Imaging 49:23–40. https://doi.org/10.1002/jmri.26293
Du S, Gao S, Zhang L, Yang X, Qi X, Li S (2021) Improved discrimination of molecular subtypes in invasive breast cancer: comparison of multiple quantitative parameters from breast MRI. Magn Reson Imaging 77:148–158. https://doi.org/10.1016/j.mri.2020.12.001
Roknsharifi S, Fishman MDC, Agarwal MD, Brook A, Kharbanda V, Dialani V (2019) The role of diffusion weighted imaging as supplement to dynamic contrast enhanced breast MRI: can it help predict malignancy, histologic grade and recurrence? Acad Radiol 26:923–929. https://doi.org/10.1016/j.acra.2018.09.003
Park SH, Choi HY, Hahn SY (2015) Correlations between apparent diffusion coefficient values of invasive ductal carcinoma and pathologic factors on diffusion-weighted MRI at 3.0 Tesla. J Magn Reson Imaging 41:175–182. https://doi.org/10.1002/jmri.24519
Kim JJ, Kim JY, Suh HB et al (2022) Characterization of breast cancer subtypes based on quantitative assessment of intratumoral heterogeneity using dynamic contrast-enhanced and diffusion-weighted magnetic resonance imaging. Eur Radiol 32:822–833. https://doi.org/10.1007/s00330-021-08166-4
Shukla-Dave A, Obuchowski NA, Chenevert TL et al (2019) Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J Magn Reson Imaging 49:e101–e121. https://doi.org/10.1002/jmri.26518
Funding
This study was supported by the National Natural Science Foundation of China (82102130, 12126610), Guangdong Basic and Applied Basic Research Foundation (2021A1515010385, 2023A1515011305), Guangzhou Basic and Applied Basic Research Foundation (2023A04J2112), and SKY Imaging Research Fund Project of China International Medical Foundation (Z-2014-07-1912-21).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jun Shen.
Conflict of interest
Two of the authors (Mengzhu Wang and Xu Yan) are employees of Siemens Healthcare.
The other authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
All participants provided written informed consent.
Ethical approval
Institutional Review Board approval was obtained from the Institutional Review Board of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University (Guangzhou, China) (SYSEC-KY-KS-2022-027).
Study subjects or cohorts overlap
No study subject or cohort has been previously reported in this study.
Methodology
• prospective
• diagnostic study
• single-center study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mao, C., Hu, L., Jiang, W. et al. Discrimination between human epidermal growth factor receptor 2 (HER2)-low-expressing and HER2-overexpressing breast cancers: a comparative study of four MRI diffusion models. Eur Radiol 34, 2546–2559 (2024). https://doi.org/10.1007/s00330-023-10198-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10198-x