Abstract
Purpose
To evaluate diagnostic performance and image quality of ultralow-dose CT (ULDCT) in diagnosing acute appendicitis with an image-based deep-learning denoising algorithm (IDLDA).
Methods
This retrospective multicenter study included 180 patients (mean ± standard deviation, 29 ± 9 years; 91 female) who underwent contrast-enhanced 2-mSv CT for suspected appendicitis from February 2014 to August 2016. We simulated ULDCT from 2-mSv CT, reducing the dose by at least 50%. Then we applied an IDLDA on ULDCT to produce denoised ULDCT (D-ULDCT). Six radiologists with different experience levels (three board-certified radiologists and three residents) independently reviewed the ULDCT and D-ULDCT. They rated the likelihood of appendicitis and subjective image qualities (subjective image noise, diagnostic acceptability, and artificial sensation). One radiologist measured image noise, signal-to-noise ratio (SNR), and contrast-to-noise ratio (CNR). We used the receiver operating characteristic (ROC) analyses, Wilcoxon’s signed-rank tests, and paired t-tests.
Results
The area under the ROC curves (AUC) for diagnosing appendicitis ranged 0.90–0.97 for ULDCT and 0.94–0.97 for D-ULDCT. The AUCs of two residents were significantly higher on D-ULDCT (AUC difference = 0.06 [95% confidence interval, 0.01–0.11; p = .022] and 0.05 [0.00–0.10; p = .046], respectively). D-ULDCT provided better subjective image noise and diagnostic acceptability to all six readers. However, the response of board-certified radiologists and residents differed in artificial sensation (all p ≤ .003). D-ULDCT showed significantly lower image noise, higher SNR, and higher CNR (all p < .001).
Conclusion
An IDLDA can provide better ULDCT image quality and enhance diagnostic performance for less-experienced radiologists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the technological advancements in CT imaging, the use of CT scans has rapidly increased worldwide [1, 2]. Concerns regarding the potential cancer risks implicated with radiation exposure from CT scans have also been raised, estimated to contribute to 1.5 to 2.0% of all cancers diagnosed in the United States [3]. Exposure to even a single abdominopelvic CT for diagnosing appendicitis scan may increase the risk of developing hematologic malignant neoplasms [4]. The imaging community has made several efforts to reduce the dose to as low as reasonably achievable; however, physicians and radiologists considered the trade-off of lower image quality unsatisfactory.
CT vendors have implemented various technologies to reduce radiation doses while maintaining image quality, such as automatic exposure control, noise reduction filters, and iterative reconstruction algorithms [5,6,7]. While iterative reconstruction played a critical role in preserving or enhancing image quality in low-dose CT examinations, it has limitations of being vendor-specific and not able to apply CT images generally. An alternative approach involves using vendor-agnostic image-based noise reduction strategies [8, 9]. Image-based methods are relatively inexpensive to install and can be useful in CT practices that employ multiple different scanner models [10], as a single server can service various CT scanners. Furthermore, recent advancements in deep-learning techniques have facilitated improvements in image-based denoising strategies, making them more versatile and applicable.
Compelling evidence suggests that CT can be used to diagnose appendicitis at a radiation level as low as 2 mSv, without negative ramifications [11,12,13]. In this study, we aimed to evaluate the diagnostic performance and image quality of 1-mSv CT (hereinafter, ultralow-dose CT [ULDCT]) with an image-based deep-learning denoising algorithm in patients with suspected appendicitis.
Materials and methods
Study design and participants
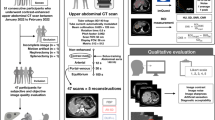
The institutional review board approved this retrospective study, and the requirement for informed consent was waived. We extracted clinical data and CT images of patients from a previous multicenter trial (Low-dOse CT for Appendicitis Trial, LOCAT; ClinicalTrials.gov number, NCT01925014) that demonstrated the non-inferiority of low-dose CT (with a target effective dose of 2 mSv) compared to standard-dose CT (with a target effective dose less than 8 mSv) in the diagnosis of appendicitis in adolescents and young adults [11]. The eligibility criteria for the trial were patients aged 15–44 years who were referred from the emergency departments for CT examination under the suspicion of appendicitis. The final diagnosis of appendicitis is based on the trial data, including surgical, pathologic, and follow-up results. In this study, we included 180 patients (15 to 44 years of age; 91 female) who underwent 2-mSv CT examinations from February 2014 to August 2016. We randomly selected 15 patients per each type of CT machine (Fig. 1). The characteristics of the study population are summarized in Table 1. We wrote this report in line with a reporting guideline (Standards for Reporting of Diagnostic Accuracy; STARD) [14].
CT image acquisition
The patients underwent 2-mSv CT from machines with 16 to 640 channels from various manufacturers (64 and 128 channels from GE Healthcare; 16, 64, 128, and 256 from Philips; 16, 64, 128, and 128 (64 × 2) from Siemens; 64 and 640 from Toshiba). All patients underwent CT scans using intravenous contrast agents. The abdominopelvic CT images were obtained during the portal venous phase and reconstructed using filtered back projection with a slice thickness of 4 mm and a slice interval of 3 mm. The mean volumetric CT dose index was 2.6 ± 0.8 mGy, and the mean dose-length product was 139.3 ± 45.7 mGy·cm.
Simulation of ultralow-dose CT and application of denoising algorithm
From the 2-mSv CT images, we simulated ULDCT images with an image-based reduced-dose CT simulation technique [15], reducing the dose by at least 50%. This technique is based on sinogram synthesis and image reconstruction using only CT images while not requiring raw sinogram data. Previous studies validated the dose reduction technique, which provided realistic low-dose images including the noise and textual appearance [16,17,18].
Then, we used an image-based deep-learning denoising algorithm (ClariCT.AI™, ClariPI) to generate denoised ULDCT (D-ULDCT) images [19]. This deep-learning algorithm is a vendor-neutral image reconstruction technique based on a modified U-net type convolutional neural network model [20]. The algorithm was trained using a dataset of over 1 million CT images, covering 2,100 combinations of scan and reconstruction conditions, including variations in kVp, mAs, automatic exposure control, slice thickness, contrast enhancement, and convolution kernels. The dataset encompassed 24 scanner models from four CT manufacturers (GE Healthcare, Siemens, Philips, and Canon). The algorithm’s performance has been validated in several studies [17, 21,22,23,24].
Qualitative image analysis
Six radiologists with different experience levels (three board-certified abdominal radiologists with 6 to 7 years of clinical experience, one third-year, and two second-year residents) independently reviewed the ULDCT and D-ULDCT images. We randomly assigned 180 patients to two groups (90 patients in groups A and B, respectively). In the first session, each radiologist assessed the ULDCT images of Group A and the D-ULDCT images of Group B. In the second session, they evaluated the D-ULDCT images of Group A and the ULDCT images of Group B. To reduce recall bias, the first and second sessions were separated with a washout period of at least 4 weeks, and the order of the CT images was randomized and different for each session. All readers were informed that patients underwent CT examinations for suspected appendicitis but were blinded to other patient information, study date, radiation dose, and reconstruction algorithm.
Firstly, the radiologists were asked to rate appendiceal visualization and likelihood score for appendicitis using the standardized CT report form (Supplementary Table 1). Appendiceal visualization was rated using a 3-point Likert scale (grade 0, not identified; grade 1, unclearly or partially visualized; and grade 2, clearly and entirely visualized). If the CT image showed phlegm or abscess with clear continuity with the remaining appendiceal base, grade 2 was assigned. The likelihood score for appendicitis was rated on a 5-point Likert scale. The primary diagnostic criterion was appendiceal enlargement (larger than 6 mm in diameter) with mural thickening and periappendiceal fat stranding. Secondary diagnostic criteria were abnormal mural enhancement, appendicolith, phlegmon, and abscess. For diagnostic sensitivity and specificity calculation, the likelihood score for appendicitis ≥ 3 was considered positive for the diagnosis [25].
Secondly, the radiologists independently rated the image quality of ULDCT and D-ULDCT by using a 5-point Likert scale (Supplementary Table 2). The following attributes were evaluated: subjective image noise (defined as the degree of mottling or graininess in the images), diagnostic acceptability (defined as the reader’s confidence in making a reasonable diagnosis from the image), and artificial sensation (defined as the degree of plastic-looking, smooth, paint-brushed, or unnatural texture).
Quantitative image analysis
A quantitative analysis of the image quality was conducted based on image noise, signal-to-noise ratio (SNR), and contrast-to-noise ratio (CNR) [26]. A board-certified radiologist with 7 years of abdominal imaging experience, who was independent of the six readers, measured the mean Hounsfield unit and standard deviation (SD) of the hepatic parenchyma, paraspinal muscle, abdominal aorta, and subcutaneous fat on ULDCT and D-ULDCT images using oval-shaped regions of interest (ROIs; 50 to 200 mm2 in size). Hounsfield unit of the hepatic parenchyma was obtained by averaging values of the four liver sections (left lateral, left medial, right anterior, and right posterior). The ROIs were placed in homogeneous areas at the level of the umbilical portion of the left portal vein, avoiding structures such as large vessels, intramuscular fat, or calcified vessel walls. The ROIs were positioned at the same location and size on ULDCT and D-ULDCT images from the same patient.
We calculated the image noise as the mean SD of the ROIs. SNR and CNR for each target region were determined using the following equations [26]:
SNRi = ROIi / SDi.
CNRi = (ROIi - ROIfat) / SDfat.
where ROIi is the mean attenuation of the region, ROIfat is the mean attenuation of the subcutaneous fat, SDi is the image noise of the region, and SDfat is the image noise of the subcutaneous fat.
Reference standard.
In previous trial, independent outcome assessors, who were two emergency department physicians and five radiologists, adjudicated the final diagnosis of appendicitis based on the trial data, including surgical findings, pathologic findings, and follow-up results [27]. All final diagnoses of appendicitis included in this study were established based on surgical and pathologic findings [11]. Histopathologic diagnosis of appendicitis was defined as neutrophil infiltration in the appendiceal wall [28].
Statistical analysis
We used the Wilcoxon signed-rank test to compare appendiceal visualization from each reader between ULDCT and D-ULDCT. We used the receiver operating characteristic (ROC) analysis to assess the diagnostic accuracy of readers in diagnosing appendicitis. We compared the area under the receiver-operating characteristic curve (AUC) of each reader between ULDCT and D-ULDCT using DeLong’s test for two correlated ROC curves. Additionally, we calculated diagnostic sensitivity and specificity in the ULDCT and D-ULDCT. For interobserver agreement, we calculated the quadratic weighted Kappa coefficient for board-certified radiologists and residents regarding ULDCT and D-ULDCT. We used z tests to compare the interobserver agreement between ULDCT and D-ULDCT.
We used the Wilcoxon signed-rank test to compare the subjective qualitative scores between ULDCT and D-ULDCT in each reader and the paired t-test to compare quantitative parameters (image noise, SNR, and CNR) between ULDCT and D-ULDCT.
Statistical analyses were performed using R software version 3.6.3 (www.R-project.org, R Foundation for Statistical Computing). A two-sided p-value of < 0.05 was considered statistical significance.
Results
Qualitative image analysis
The appendix was well visualized (appendiceal visualization score 2) in most patients (77.8–91.7% on ULDCT and 79.4–92.2% on D-ULDCT) (Fig. 2). Appendiceal visualization scores of all readers were not significantly different between ULDCT and D-ULDCT (Supplementary Table 3).
The AUC of diagnosing appendicitis in readers ranged 0.90–0.97 and 0.94–0.97 on ULDCT and D-ULDCT, respectively (Fig. 3). The diagnostic performance of three board-certified radiologists (reader 1, reader 2, and reader 3) and one third-year resident (reader 4) for appendicitis did not differ significantly between ULDCT and D-ULDCT. However, for two second-year residents, diagnostic performance was significantly higher in D-ULDCT than in ULDCT (AUC difference of reader 5: 0.06 [95% CI, 0.01–0.11; p = .022]; AUC difference of reader 6: 0.05 [95% CI, 0.00–0.10; p = .046]) (Fig. 4; Table 2). On ULDCT, the diagnostic sensitivity of each reader ranged from 83 to 98%, and the specificity ranged from 75 to 89%. On D-ULDCT, the diagnostic sensitivity ranged from 88 to 100%, and the specificity ranged from 75 to 93% (Supplementary Table 4). The interobserver agreement for likelihood of appendicitis between ULDCT and D-ULDCT showed no significant difference in board-certified radiologists (0.87 vs. 0.86; 0.01 [95% CI, -0.05–0.07; p = .76]) and residents (0.68 vs. 0.72; -0.05 [95% CI, -0.14–0.05; p = .32]).
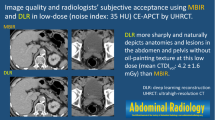
Representative images that an image-based deep-learning denoising algorithm improved diagnostic accuracy. A 37-year-old woman underwent a CT examination for suspected appendicitis (64-channel CT from Siemens). A second-year radiology resident (reader 6) diagnosed terminal ileitis on ultralow-dose CT images (a, c) and appendicitis on ultralow-dose CT with deep-learning denoising algorithm (b, d). The patient was confirmed with appendicitis through an appendectomy
All readers evaluated that D-ULDCT images using the image-based deep-learning denoising algorithm had lesser subjective image noise and better diagnostic acceptability (p < .001; except p-value of reader 3 on diagnostic acceptability = 0.003, Table 3). Regarding the artificial sensation, the evaluation from three board-certified radiologists indicated that D-ULDCT images were perceived as more artificial. Contrarily, the assessment from three radiology residents suggested that D-ULDCT images appeared more natural (p < .001).
Quantitative image analysis
D-ULDCT images showed significantly lower image noise, higher SNR, and higher CNR (p < .001) compared to ULDCT images (Table 4). The image noise was lower on D-ULDCT than on ULDCT for the hepatic parenchyma (17.3 ± 6.5 vs. 42.0 ± 15.1, p < .001), paraspinal muscle (15.9 ± 6.0 vs. 37.4 ± 13.4, p < .001), and abdominal aorta (21.5 ± 8.0 vs. 50.0 ± 17.9, p < .001). The SNR was higher on D-ULDCT than on ULDCT for the hepatic parenchyma (8.6 ± 3.2 vs. 3.5 ± 1.3, p < .001), paraspinal muscle (4.9 ± 1.6 vs. 2.1 ± 0.7, p < .001), and abdominal aorta (10.1 ± 4.1 vs. 4.3 ± 1.7, p < .001). The CNR was higher on D-ULDCT than on ULDCT for the hepatic parenchyma (16.4 ± 5.8 vs. 8.1 ± 2.5, p < .001), paraspinal muscle (12.0 ± 4.1 vs. 5.9 ± 1.8, p < .001), and abdominal aorta (20.6 ± 7.5 vs. 10.1 ± 3.3, p < .001). The image noise seemed to increase and SNR/CNR seemed to decrease, according to body mass index (Supplementary Table 5).
Discussion
In this study, an image-based deep-learning denoising algorithm improved image quality of ultralow-dose CT in diagnosing acute appendicitis and the diagnostic performance of less-experienced radiology residents.
Our study demonstrates several strengths in managing dose reduction in CT, which is a challenging situation in clinical practice. First, our analysis applies the image-based deep-learning algorithm to reconstructed CT images, not sinograms, ensuring generalizability. Previous studies have reported improvements in qualitative or quantitative features on CT images with deep learning-based reconstruction algorithms compared to filtered back projection or iterative reconstruction techniques [9, 29,30,31,32]. However, most of these studies relied on sinograms for applying deep learning-based reconstruction. In contrast, our study used image-based denoising algorithm, providing a practical approach to dose reduction even in cases where sinograms cannot be obtained. We attributed this capability to the denoising algorithm trained on a large dataset of image data. Previous studies demonstrated its ability to improve image quality and diagnostic accuracy in low-dose coronary CT angiography [17], show the best overall image quality with fewer artifacts in ultralow-dose chest CT when compared with vendor-specific deep learning image reconstruction [23], and maintain image quality while reducing radiation dose and iodine administration in pediatric abdominal CT examinations [24]. Second, we analyzed image data from various CT machines from different vendors. Previous feasibility study with 30 patients had reported that ULDCT would be non-inferior to 2-mSv CT for diagnosing appendicitis [33]. However, the study had limitations, including a small sample size with only one CT machine. On the other hand, our results demonstrated promising applicability, versatility, and the potential for broad implementation across larger patients and various CT machines. Third, we revealed the usefulness of the image-based deep learning denoising algorithm in the clinical task of diagnosing appendicitis. This aspect goes beyond a simple image quality comparison between ULDCT and D-ULDCT. There might be a certain gap between improving image quality and assisting diagnosis. Thus, our findings showed that the practical application of the deep-learning denoising algorithm could help enhance diagnostic accuracy, especially for less-experienced radiologists.
Interestingly, in terms of appendiceal visualization, we did not observe a significant difference between ULDCT and D-ULDCT. The appendix was well visualized in most patients on both the ULDCT and D-ULDCT images, with a range of 77.8–91.7% for ULDCT and 79.4–92.2% for D-ULDCT, respectively. Considering that a previous study achieved good visualization in more than 85% of cases with 2-mSv CT [34], appendiceal visualization might be slightly compromised, although the formal statistical comparison is limited. However, regardless of the degree of appendix visualization, radiologists achieved a good diagnostic performance (AUC, 0.94–0.97) for appendicitis in D-ULDCT, which aligns with the previous study’s findings [34]. Intriguingly, radiologists could accurately diagnose or rule out appendicitis in most cases, even if the appendix was not visualized. While we cannot provide a definitive explanation for this finding, we assume that radiologists possibly relied on secondary findings, such as fat infiltration or fluid collection in the right lower quadrant of the abdomen, to aid in their diagnostic decision-making.
Regarding the artificial sensation in denoised images, board-certified abdominal radiologists perceived D-ULDCT as more artificial, whereas radiology residents found it more natural. We hypothesized that the images from D-ULDCT might be less familiar and more artificial to experienced radiologists who are accustomed to reading CT images with filtered back projection or iterative reconstruction. On the other hand, D-ULDCT might seem more clear and natural to less experienced residents compared to ULDCT, and this partially contributed to the finding that D-ULDCT improved the diagnostic accuracies of the two residents.
Our study had limitations. First, ultralow-dose CT images were simulated from the 2-mSv CT images using a validated noise insertion tool [15]. While this allowed us to compare images from the same patients at different radiation doses without additional exposure, it is important to note that the simulated images may not perfectly replicate the original data. Thus, the results obtained from the simulated images may not completely reflect the outcomes observed in actual CT examinations. Second, although we drew the ROI for various organs for quantitative analysis, there may be potential bias in measuring the mean Hounsfield unit, particularly for small objects. Third, our study focused solely on evaluating the deep learning denoising algorithm and did not include a comparison with other CT reconstruction methods, such as filtered back projection or iterative reconstruction. Future studies could benefit from including such comparisons to provide a more comprehensive understanding of the performance and effectiveness of different reconstruction techniques.
In conclusion, an image-based deep-learning denoising algorithm can provide better image quality of ultralow-dose CT in diagnosing acute appendicitis and enhance diagnostic performance for less-experienced radiologists.
References
Mahesh M, Ansari AJ, Mettler FA Jr. (2023) Patient exposure from radiologic and nuclear medicine procedures in the United States and worldwide: 2009–2018. Radiology 307: e239006. https://doi.org/10.1148/radiol.239006
Smith-Bindman R, Kwan ML, Marlow EC, Theis MK, Bolch W, Cheng SY, Bowles EJA, Duncan JR, Greenlee RT, Kushi LH, Pole JD, Rahm AK, Stout NK, Weinmann S, Miglioretti DL (2019) Trends in use of medical imaging in US health care systems and in Ontario, Canada, 2000–2016. JAMA 322: 843–856. https://doi.org/10.1001/jama.2019.11456
Brenner DJ, Hall EJ (2007) Computed tomography–an increasing source of radiation exposure. N Engl J Med 357: 2277–2284. https://doi.org/10.1056/NEJMra072149
Lee KH, Lee S, Park JH, Lee SS, Kim HY, Lee WJ, Cha ES, Kim KP, Lee W, Lee JY, Lee KH (2021) Risk of hematologic malignant neoplasms from abdominopelvic computed tomographic radiation in patients who underwent appendectomy. JAMA Surg 156: 343–351. https://doi.org/10.1001/jamasurg.2020.6357
Yu L, Liu X, Leng S, Kofler JM, Ramirez-Giraldo JC, Qu M, Christner J, Fletcher JG, McCollough CH (2009) Radiation dose reduction in computed tomography: techniques and future perspective. Imaging Med 1: 65–84. https://doi.org/10.2217/iim.09.5
McCollough CH, Primak AN, Braun N, Kofler J, Yu L, Christner J (2009) Strategies for reducing radiation dose in CT. Radiol Clin North Am 47: 27–40. https://doi.org/10.1016/j.rcl.2008.10.006
Del Gaizo AJ, Fletcher JG, Yu L, Paden RG, Spencer GC, Leng S, Silva AM, Fidler JL, Silva AC, Hara AK (2013) Reducing radiation dose in CT enterography. Radiographics 33: 1109–1124. https://doi.org/10.1148/rg.334125074
Kim Y, Oh DY, Chang W, Kang E, Ye JC, Lee K, Kim HY, Kim YH, Park JH, Lee YJ, Lee KH (2021) Deep learning-based denoising algorithm in comparison to iterative reconstruction and filtered back projection: a 12-reader phantom study. Eur Radiol 31: 8755–8764. https://doi.org/10.1007/s00330-021-07810-3
Shin YJ, Chang W, Ye JC, Kang E, Oh DY, Lee YJ, Park JH, Kim YH (2020) Low-dose abdominal CT using a deep learning-based denoising algorithm: a comparison with CT reconstructed with filtered back projection or iterative reconstruction algorithm. Korean J Radiol 21: 356–364. https://doi.org/10.3348/kjr.2019.0413
Fletcher JG, Hara AK, Fidler JL, Silva AC, Barlow JM, Carter RE, Bartley A, Shiung M, Holmes DR, 3rd, Weber NK, Bruining DH, Yu L, McCollough CH (2015) Observer performance for adaptive, image-based denoising and filtered back projection compared to scanner-based iterative reconstruction for lower dose CT enterography. Abdom Imaging 40: 1050–1059. https://doi.org/10.1007/s00261-015-0384-1
LOCAT Group (2017) Low-dose CT for the diagnosis of appendicitis in adolescents and young adults (LOCAT): a pragmatic, multicentre, randomised controlled non-inferiority trial. Lancet Gastroenterol Hepatol 2: 793–804. https://doi.org/10.1016/s2468-1253(17)30247-9
Rud B, Vejborg TS, Rappeport ED, Reitsma JB, Wille-Jørgensen P (2019) Computed tomography for diagnosis of acute appendicitis in adults. Cochrane Database Syst Rev 2019: CD009977. https://doi.org/10.1002/14651858.CD009977.pub2
Kim K, Kim YH, Kim SY, Kim S, Lee YJ, Kim KP, Lee HS, Ahn S, Kim T, Hwang SS, Song KJ, Kang SB, Kim DW, Park SH, Lee KH (2012) Low-dose abdominal CT for evaluating suspected appendicitis. N Engl J Med 366: 1596–1605. https://doi.org/10.1056/NEJMoa1110734
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, Lijmer JG, Moher D, Rennie D, de Vet HC, Kressel HY, Rifai N, Golub RM, Altman DG, Hooft L, Korevaar DA, Cohen JF (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Radiology 277: 826–832. https://doi.org/10.1148/radiol.2015151516
Kim CW, Kim JH (2014) Realistic simulation of reduced-dose CT with noise modeling and sinogram synthesis using DICOM CT images. Med Phys 41: 011901. https://doi.org/10.1118/1.4830431
Afat S, Brockmann C, Nikoubashman O, Müller M, Thierfelder KM, Brockmann MA, Nikolaou K, Wiesmann M, Kim JH, Othman AE (2018) Diagnostic accuracy of simulated low-dose perfusion CT to detect cerebral perfusion impairment after aneurysmal subarachnoid hemorrhage: a retrospective analysis. Radiology 287: 643–650. https://doi.org/10.1148/radiol.2017162707
Hong JH, Park EA, Lee W, Ahn C, Kim JH (2020) Incremental image noise reduction in coronary CT angiography using a deep learning-based technique with iterative reconstruction. Korean J Radiol 21: 1165–1177. https://doi.org/10.3348/kjr.2020.0020
Storz C, Kolb M, Kim JH, Weiss J, Kunz WG, Nikolaou K, Bamberg F, Othman AE (2018) Impact of radiation dose reduction in abdominal computed tomography on diagnostic accuracy and diagnostic performance in patients with suspected appendicitis: an intraindividual comparison. Acad Radiol 25: 309–316. https://doi.org/10.1016/j.acra.2017.09.012
U.S. Food and Drug Administration. 510(k) Premarket Notification (device name ClariCT.AI). FDA Web. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=K183460. Accessed August 13, 2023.
Ahn CK, Heo C, Kim JH. Combined low-dose simulation and deep learning for CT denoising: application in ultra-low-dose chest CT. Published 27 March 2019: SPIE; 2019. p. 1094846. https://doi.org/10.1117/12.2521539
Lim WH, Choi YH, Park JE, Cho YJ, Lee S, Cheon JE, Kim WS, Kim IO, Kim JH (2019) Application of vendor-neutral iterative reconstruction technique to pediatric abdominal computed tomography. Korean J Radiol 20: 1358–1367. https://doi.org/10.3348/kjr.2018.0715
Kolb M, Storz C, Kim JH, Weiss J, Afat S, Nikolaou K, Bamberg F, Othman AE (2019) Effect of a novel denoising technique on image quality and diagnostic accuracy in low-dose CT in patients with suspected appendicitis. Eur J Radiol 116: 198–204. https://doi.org/10.1016/j.ejrad.2019.04.026
Nam JG, Ahn C, Choi H, Hong W, Park J, Kim JH, Goo JM (2021) Image quality of ultralow-dose chest CT using deep learning techniques: potential superiority of vendor-agnostic post-processing over vendor-specific techniques. Eur Radiol 31: 5139–5147. https://doi.org/10.1007/s00330-020-07537-7
Lee S, Choi YH, Cho YJ, Lee SB, Cheon JE, Kim WS, Ahn CK, Kim JH (2021) Noise reduction approach in pediatric abdominal CT combining deep learning and dual-energy technique. Eur Radiol 31: 2218–2226. https://doi.org/10.1007/s00330-020-07349-9
Daly CP, Cohan RH, Francis IR, Caoili EM, Ellis JH, Nan B (2005) Incidence of acute appendicitis in patients with equivocal CT findings. AJR Am J Roentgenol 184: 1813–1820. https://doi.org/10.2214/ajr.184.6.01841813
Park J, Shin J, Min IK, Bae H, Kim YE, Chung YE (2022) Image quality and lesion detectability of lower-dose abdominopelvic CT obtained using deep learning image reconstruction. Korean J Radiol 23: 402–412. https://doi.org/10.3348/kjr.2021.0683
Ahn S (2014) LOCAT (low-dose computed tomography for appendicitis trial) comparing clinical outcomes following low- vs standard-dose computed tomography as the first-line imaging test in adolescents and young adults with suspected acute appendicitis: study protocol for a randomized controlled trial. Trials 15: 28. https://doi.org/10.1186/1745-6215-15-28
Lamps LW. Appendix. 11th ed. Goldblum JR, Lamps LW, McKenney JK, Myers JL, Ackerman LV, Rosai J, editors. Philadelphia, PA: Elsevier; 2018. pp 617–647
Deák Z, Grimm JM, Treitl M, Geyer LL, Linsenmaier U, Körner M, Reiser MF, Wirth S (2013) Filtered back projection, adaptive statistical iterative reconstruction, and a model-based iterative reconstruction in abdominal CT: an experimental clinical study. Radiology 266: 197–206. https://doi.org/10.1148/radiol.12112707
Mohammadinejad P, Mileto A, Yu L, Leng S, Guimaraes LS, Missert AD, Jensen CT, Gong H, McCollough CH, Fletcher JG (2021) CT noise-reduction methods for lower-dose scanning: strengths and weaknesses of iterative reconstruction algorithms and new techniques. Radiographics 41: 1493–1508. https://doi.org/10.1148/rg.2021200196
Park S, Yoon JH, Joo I, Yu MH, Kim JH, Park J, Kim SW, Han S, Ahn C, Kim JH, Lee JM (2022) Image quality in liver CT: low-dose deep learning vs standard-dose model-based iterative reconstructions. Eur Radiol 32: 2865–2874. https://doi.org/10.1007/s00330-021-08380-0
Son W, Kim M, Hwang JY, Kim YW, Park C, Choo KS, Kim TU, Jang JY (2022) Comparison of a deep learning-based reconstruction algorithm with filtered back projection and iterative reconstruction algorithms for pediatric abdominopelvic CT. Korean J Radiol 23: 752–762. https://doi.org/10.3348/kjr.2021.0466
Park JH, Jeon JJ, Lee SS, Dhanantwari AC, Sim JY, Kim HY, Lee KH (2018) Can we perform CT of the appendix with less than 1 mSv? a de-escalating dose-simulation study. Eur Radiol 28: 1826–1834. https://doi.org/10.1007/s00330-017-5159-3
Cho J, Kim Y, Lee S, Min HD, Ko Y, Chee CG, Kim HY, Park JH, Lee KH (2022) Appendiceal visualization on 2-mSv CT vs. conventional-dose CT in adolescents and young adults with suspected appendicitis: an analysis of large pragmatic randomized trial data. Korean J Radiol 23: 413–425. https://doi.org/10.3348/kjr.2021.0504
Acknowledgements
The data in our study were obtained from the database of a randomized controlled trial by the LOCAT Group.
Funding
This study has received funding by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2022R1A2B5B01001804) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI20C2092) and Seoul National University Bundang Hospital Research Fund (14-2022-0021).
Open Access funding enabled and organized by Seoul National University Hospital.
Author information
Authors and Affiliations
Contributions
HUC and JC conceived and designed the study. HUC and JC drafted the initial manuscript and acquired data. JH and SL performed statistical analyses. All authors critically reviewed and revised the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The institutional review board approved this retrospective study, and the requirement for informed consent was waived.
Conflict of interest
Jungheum Cho received a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute and Seoul National University Bundang Hospital. Kyoung Ho Lee received a research grant from the National Research Foundation of Korea. The funders had no role in study design, data analysis, or preparation of the manuscript. Other authors declare there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, H.U., Cho, J., Hwang, J. et al. Diagnostic performance and image quality of an image-based denoising algorithm applied to radiation dose-reduced CT in diagnosing acute appendicitis. Abdom Radiol 49, 1839–1849 (2024). https://doi.org/10.1007/s00261-024-04246-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-024-04246-3