Abstract
Objective
To investigate the prognostic value of the diffusion kurtosis imaging (DKI)-derived parameters D value, K value, diffusion-weighted imaging (DWI) parameter apparent diffusion coefficient (ADC) value, and magnetic resonance imaging (MRI)-detected extramural venous invasion (EMVI) (mrEMVI) in rectal cancer patients.
Methods
Forty patients who underwent MRI for rectal cancer were retrospectively evaluated. DKI-derived parameters D and K were measured using the Medical Imaging Interaction Toolkit. Conventional ADC values were measured from the corresponding DWI images. An experienced radiologist evaluated the mrEMVI status on MR images using the mrEMVI scoring system. An independent sample t-test or analysis of variance was used to analyze and compare the measurement data. The x2 test or Fisher exact test was used for categorical variables. Receiver operating characteristic curves were used to assess the diagnostic performance of these parameters.
Results
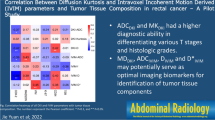
Among the 40 patients, MRI showed positive EMVI in 15 patients and negative EMVI in 25 patients. Positive mrEMVI status was associated with age, positive circumferential resection margin, pT-stage, lymphovascular invasion (LVI), distant metastasis, and serum carcinoembryonic antigen (CEA) level (P = 0.004–0.036). The dispersion coefficient (D) values and ADC values were significantly higher in the mucinous adenocarcinoma (MC) group than in the common adenocarcinoma (AC) group (P = 0.001), while kurtosis coefficient (K) values were lower in the MC group than in the AC group (P = 0.022). D values were significantly higher in the KRAS-mutated group than in the wild-type group (P < 0.05), whereas K values were lower in the KRAS-mutated group than in the wild-type group (P < 0.05). All three parameters (D, K, and ADC values) showed good diagnostic performance for discriminating MC from AC. Both the D and K values showed certain diagnostic performance for discriminating KRAS mutation.
Conclusion
DKI-derived parameters, conventional ADC values, and mrEMVI are associated with different histopathological prognostic factors. All DKI-derived parameters and conventional ADC values may distinguish MC from AC. DKI-derived parameters may also be used to discriminate KRAS mutation.
Graphical abstract






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022). Cancer statistics, 2022. CA Cancer J Clin 72(1):7-33. https://doi.org/10.3322/caac.21708.
National Health Commission of the People’s Republic of C (2020). [Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer (2020 edition)]. Zhonghua Wai Ke Za Zhi 58(8):561-85. https://doi.org/10.3760/cma.j.cn112139-20200518-00390.
Brown G, Richards CJ, Newcombe RG et al. (1999). Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology 211(1):215-22. https://doi.org/10.1148/radiology.211.1.r99ap35215.
Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ (2019). MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics 39(2):367-87. https://doi.org/10.1148/rg.2019180114.
Beets-Tan RG, Beets GL, Vliegen RF et al. (2001). Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet 357(9255):497-504. https://doi.org/10.1016/s0140-6736(00)04040-x.
Beets-Tan RG, Beets GL (2014). MRI for assessing and predicting response to neoadjuvant treatment in rectal cancer. Nat Rev Gastroenterol Hepatol 11(8):480-8. https://doi.org/10.1038/nrgastro.2014.41.
Keller DS, Berho M, Perez RO, Wexner SD, Chand M (2020). The multidisciplinary management of rectal cancer. Nat Rev Gastroenterol Hepatol 17(7):414-29. https://doi.org/10.1038/s41575-020-0275-y.
Nagtegaal ID, Quirke P (2008). What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 26(2):303-12. https://doi.org/10.1200/JCO.2007.12.7027.
Agger E, Jorgren F, Lydrup ML, Buchwald P (2021). Circumferential Resection Margin is Associated with Distant Metastasis After Rectal Cancer Surgery: A Nation-Wide Population-Based Study Cohort. Ann Surg. https://doi.org/10.1097/SLA.0000000000005302.
Group MS (2006). Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 333(7572):779. https://doi.org/10.1136/bmj.38937.646400.55.
Horn A, Dahl O, Morild I (1991). Venous and neural invasion as predictors of recurrence in rectal adenocarcinoma. Dis Colon Rectum 34(9):798-804. https://doi.org/10.1007/BF02051074.
Tripathi P, Rao SX, Zeng MS (2017). Clinical value of MRI-detected extramural venous invasion in rectal cancer. J Dig Dis 18(1):2-12. https://doi.org/10.1111/1751-2980.12439.
Sohn B, Lim JS, Kim H et al. (2015). MRI-detected extramural vascular invasion is an independent prognostic factor for synchronous metastasis in patients with rectal cancer. Eur Radiol 25(5):1347-55. https://doi.org/10.1007/s00330-014-3527-9.
Zhang XY, Wang S, Li XT et al. (2018). MRI of Extramural Venous Invasion in Locally Advanced Rectal Cancer: Relationship to Tumor Recurrence and Overall Survival. Radiology 289(3):677-85. https://doi.org/10.1148/radiol.2018172889.
Zhao L, Liang M, Yang Y, Zhang H, Zhao X (2021). Prediction of false-negative extramural venous invasion in patients with rectal cancer using multiple mathematical models of diffusion-weighted imaging. Eur J Radiol 139:109731. https://doi.org/10.1016/j.ejrad.2021.109731.
Fornell-Perez R, Vivas-Escalona V, Aranda-Sanchez J et al. (2020). Primary and post-chemoradiotherapy MRI detection of extramural venous invasion in rectal cancer: the role of diffusion-weighted imaging. Radiol Med 125(6):522-30. https://doi.org/10.1007/s11547-020-01137-7.
Enkhbaatar NE, Inoue S, Yamamuro H et al. (2018). MR Imaging with Apparent Diffusion Coefficient Histogram Analysis: Evaluation of Locally Advanced Rectal Cancer after Chemotherapy and Radiation Therapy. Radiology 288(1):129-37. https://doi.org/10.1148/radiol.2018171804.
Dzik-Jurasz A, Domenig C, George M et al. (2002). Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 360(9329):307-8. https://doi.org/10.1016/S0140-6736(02)09520-X.
Zhu L, Pan Z, Ma Q et al. (2017). Diffusion Kurtosis Imaging Study of Rectal Adenocarcinoma Associated with Histopathologic Prognostic Factors: Preliminary Findings. Radiology 284(1):66-76. https://doi.org/10.1148/radiol.2016160094.
Weiser MR (2018). AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol 25(6):1454-5. https://doi.org/10.1245/s10434-018-6462-1.
Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G (2008). Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg 95(2):229-36. https://doi.org/10.1002/bjs.5917.
Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT (2003). Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg 90(3):355-64. https://doi.org/10.1002/bjs.4034.
Gu C, Yang X, Zhang X et al. (2019). The prognostic significance of MRI-detected extramural venous invasion, mesorectal extension, and lymph node status in clinical T3 mid-low rectal cancer. Sci Rep 9(1):12523. https://doi.org/10.1038/s41598-019-47466-0.
Ahn JH, Kim SH, Son JH, Jo SJ (2019). Added value of diffusion-weighted imaging for evaluation of extramural venous invasion in patients with primary rectal cancer. Br J Radiol 92(1096):20180821. https://doi.org/10.1259/bjr.20180821.
Wen Z, Chen Y, Yang X et al. (2019). Application of magnetic resonance diffusion kurtosis imaging for distinguishing histopathologic subtypes and grades of rectal carcinoma. Cancer Imaging 19(1):8. https://doi.org/10.1186/s40644-019-0192-x.
Cui Y, Cui X, Yang X et al. (2019). Diffusion kurtosis imaging-derived histogram metrics for prediction of KRAS mutation in rectal adenocarcinoma: Preliminary findings. J Magn Reson Imaging 50(3):930-9. https://doi.org/10.1002/jmri.26653.
Cui Y, Yang X, Du X, Zhuo Z, Xin L, Cheng X (2018). Whole-tumour diffusion kurtosis MR imaging histogram analysis of rectal adenocarcinoma: Correlation with clinical pathologic prognostic factors. Eur Radiol 28(4):1485-94. https://doi.org/10.1007/s00330-017-5094-3.
Funding
None.
Author information
Authors and Affiliations
Contributions
CT and PJW contributed to the study conception and design. All authors collected the data and performed the data analysis. All authors contributed to the interpretation of the data and the completion of figures and tables. All authors contributed to the drafting of the article and final approval of the submitted version.
Corresponding author
Ethics declarations
Competing interests
All the authors declare that they have no competing interests.
Ethical approval
This retrospective study was approved by the ethics committee of Yangpu Hospital of Tongji University (Shanghai, China).
Consent to participate
The requirement for informed patient consent was waived.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, C., Lu, G., Xu, J. et al. Diffusion kurtosis imaging and MRI-detected extramural venous invasion in rectal cancer: correlation with clinicopathological prognostic factors. Abdom Radiol 48, 844–854 (2023). https://doi.org/10.1007/s00261-022-03782-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03782-0