Abstract
Purpose
To explore the clinical value of contrast-enhanced computed tomography (CECT) radiomics in predicting primary site response to neoadjuvant chemotherapy in high-risk neuroblastoma.
Materials and methods
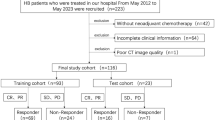
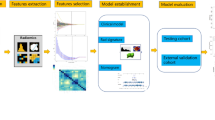
Seventy patients were retrospectively included and separated into very good partial response (VGPR) group and non-VGPR group according to the changes in primary tumor volume. The clinical features with statistical difference between the two groups were used to construct the clinical models using a logistic regression (LR) algorithm. The radiomics models based on different radiomics features selected by Kruskal–Wallis (KW) test and recursive feature elimination (RFE) were established using support vector machine (SVM) and LR algorithms. The radiomics score (Radscore) and clinical features were integrated into the combined models. Leave-one-out cross-validation (LOOCV) was used to validate the predictive performance of models in the entire dataset.
Results
The optimal clinical model achieved an area under the curve (AUC) of 0.767 [95% confidence interval (CI): 0.638, 0.896] and an accuracy of 0.771 after LOOCV. The AUCs of the best KW + SVM, KW + LR, RFE + SVM, and RFE + LR radiomics models were 0.816, 0.826, 0.853, and 0.850, respectively, and the corresponding AUCs after LOOCV were 0.780, 0.785, 0.755, and 0.772, respectively. The AUC and accuracy after LOOCV of the optimal combined model was 0.804 (95% CI: 0.694, 0.915) and 0.814, respectively. The Delong test showed a statistical difference in predictive performance between the optimal clinical and combined models after LOOCV (Z = 2.003, P = 0.045). The decision curve analysis showed that the combined model performs better than the clinical model.
Conclusion
The CECT radiomics models have a favorable predictive performance in predicting VGPR of high-risk neuroblastoma to neoadjuvant chemotherapy. When integrating radiomics features and clinical features, the predictive performance of the combined models can be further improved.
Graphical abstract







Similar content being viewed by others
Data availability
Available from the authors upon reasonable request.
References
Goldsby RE, Matthay KK (2004) Neuroblastoma: evolving therapies for a disease with many faces. Paediatr Drugs 6:107-122.
Cohn SL, Pearson AD, London WB et al (2009) The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 27:289-297.
Board PDQPTE (2002) Neuroblastoma Treatment (PDQ®): Health Professional VersionPDQ Cancer Information Summaries. National Cancer Institute (US), Bethesda (MD).
Qi Y, Zhan J (2021) Roles of Surgery in the Treatment of Patients With High-Risk Neuroblastoma in the Children Oncology Group Study: A Systematic Review and Meta-Analysis. Front Pediatr 9:706800.
Feng L, Lu X, Yang X et al (2022) An 18F-FDG PET/CT radiomics nomogram for differentiation of high-risk and non-high-risk patients of the International Neuroblastoma Risk Group Staging System. Eur J Radiol 154:110444.
Delforge X, De Cambourg P, Defachelles AS et al (2021) Unresectable thoracic neuroblastic tumors: Changes in image-defined risk factors after chemotherapy and impact on surgical management. Pediatr Blood Cancer 68:e29260.
Mansfield SA, McCarville MB, Lucas JT Jr, et al (2022) Impact of Neoadjuvant Chemotherapy on Image-Defined Risk Factors in High-Risk Neuroblastoma. Ann Surg Oncol 29:661-670.
Wang H, Chen X, Zhu J et al (2022) Changes in image-defined risk factors with neoadjuvant chemotherapy in pediatric abdominal neuroblastoma. Abdom Radiol (NY) 47:3520-3530.
Park JR, Bagatell R, Cohn SL et al (2017) Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement From the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol 35:2580-2587.
Rujkijyanont P, Photia A, Traivaree C et al (2019) Clinical outcomes and prognostic factors to predict treatment response in high risk neuroblastoma patients receiving topotecan and cyclophosphamide containing induction regimen: a prospective multicenter study. BMC Cancer 19:961.
Van Heerden J, Kruger M, Esterhuizen TM et al (2021) The Association between Tumour Markers and Meta-iodobenzylguanidine Scans in South African Children with High-risk Neuroblastoma. Clin Oncol (R Coll Radiol) 33:517-526.
Mayampurath A, Ramesh S, Michael D et al (2021) Predicting Response to Chemotherapy in Patients With Newly Diagnosed High-Risk Neuroblastoma: A Report From the International Neuroblastoma Risk Group. JCO Clin Cancer Inform 5:1181-1188.
Burnand K, Barone G, McHugh K, et al (2019) Preoperative computed tomography scanning for abdominal neuroblastomas is superior to magnetic resonance imaging for safe surgical planning. Pediatr Blood Cancer 66:e27955.
Chen AM, Trout AT, Towbin AJ (2018) A review of neuroblastoma image-defined risk factors on magnetic resonance imaging. Pediatr Radiol 48:1337-1347.
Wang H, Li T, Chen X et al (2022) Correlations Between Preoperative Radiographic Vascular Involvement of Abdominal/Pelvic Neuroblastomas on Computed Tomography and Intraoperative Vascular Injuries: Experience From a Tertiary Children's Hospital. Acad Radiol. https://doi.org/10.1016/j.acra.2022.09.010.
Horvat N, Miranda J, El Homsi M et al (2022) A primer on texture analysis in abdominal radiology. Abdom Radiol (NY) 47:2972-2985.
Wang W, Peng Y, Feng X et al (2021) Development and Validation of a Computed Tomography-Based Radiomics Signature to Predict Response to Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer. JAMA Netw Open 4:e2121143.
Xu Q, Sun Z, Li X et al (2021) Advanced gastric cancer: CT radiomics prediction and early detection of downstaging with neoadjuvant chemotherapy. Eur Radiol 31:8765-8774.
Choudhery S, Gomez-Cardona D, Favazza CP et al (2022) MRI Radiomics for Assessment of Molecular Subtype, Pathological Complete Response, and Residual Cancer Burden in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy. Acad Radiol 29 Suppl 1:S145-s154.
Tan E, Merchant K, Kn BP et al (2022) CT-based morphologic and radiomics features for the classification of MYCN gene amplification status in pediatric neuroblastoma. Childs Nerv Syst 38:1487-1495.
Liu G, Poon M, Zapala MA et al (2022) Incorporating Radiomics into Machine Learning Models to Predict Outcomes of Neuroblastoma. J Digit Imaging 35:605-612.
Chen X, Wang H, Huang K et al (2021) CT-Based Radiomics Signature With Machine Learning Predicts MYCN Amplification in Pediatric Abdominal Neuroblastoma. Front Oncol 11:687884.
Feng L, Qian L, Yang S et al (2022) Clinical parameters combined with radiomics features of PET/CT can predict recurrence in patients with high-risk pediatric neuroblastoma. BMC Med Imaging 22:102.
Yoo SY, Kim JS, Sung KW et al (2013) The degree of tumor volume reduction during the early phase of induction chemotherapy is an independent prognostic factor in patients with high-risk neuroblastoma. Cancer 119:656-664.
Matthay KK, Reynolds CP, Seeger RC et al (2009) Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children's oncology group study. J Clin Oncol 27:1007-1013.
Brodeur GM, Pritchard J, Berthold F et al (1993) Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 11:1466-1477.
Song Y, Zhang J, Zhang YD et al (2020) FeAture Explorer (FAE): A tool for developing and comparing radiomics models. PLoS One 15:e0237587.
Berthold F, Faldum A, Ernst A et al (2020) Extended induction chemotherapy does not improve the outcome for high-risk neuroblastoma patients: results of the randomized open-label GPOH trial NB2004-HR. Ann Oncol 31:422-429.
Van Heerden J, Geel J, Hendricks M et al (2020) The evaluation of induction chemotherapy regimens for high-risk neuroblastoma in South African children. Pediatr Hematol Oncol 37:300-313.
Wang H, Chen X, Liu H et al (2021) [Computed tomography-based radiomics for differential of retroperitoneal neuroblastoma and ganglioneuroblastoma in children]. Nan Fang Yi Ke Da Xue Xue Bao 41:1569-1576.
Demircioğlu A (2022) Benchmarking Feature Selection Methods in Radiomics. Invest Radiol 57:433-443.
Funding
The project was funded by Basic Research and Frontier Exploration Project (Yuzhong District, Chongqing, China) (Grant No. 20200155).
Author information
Authors and Affiliations
Contributions
Conceptualization: HW. Data curation: HW, JQ, XC, TZ. Formal analysis: HW. Investigation: HW, LZ, HD. Methodology: HW. Project administration: HW, ZP, LH. Supervision: LH. Validation: HW, JQ. Visualization: HW. Writing: HW, JQ.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
The requirement for patient informed consent was waived.
Consent for publication
Authors are responsible for correctness of the statements provided in the manuscript. The Editor-in-Chief reserves the right to reject submissions that do not meet the guidelines described in this section.
Ethical approval
This retrospective study was approved by the ethics committee of our institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haoru Wang and Jinjie Qin have been contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Qin, J., Chen, X. et al. Contrast-enhanced computed tomography radiomics in predicting primary site response to neoadjuvant chemotherapy in high-risk neuroblastoma. Abdom Radiol 48, 976–986 (2023). https://doi.org/10.1007/s00261-022-03774-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03774-0