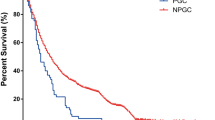
Abstract
Purpose
This study aimed to analyze the clinicopathological and computed tomography (CT) findings of papillary gastric adenocarcinoma and to evaluate the feasibility of the multivariate model based on clinical information and CT findings for discriminating papillary gastric adenocarcinomas.
Methods
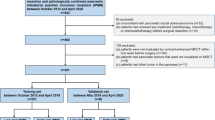
This retrospective study included 22 patients with papillary gastric adenocarcinoma and 88 patients with tubular adenocarcinoma. The demographic data, tumor markers, histopathological information, CT morphological characteristics, and CT value-related parameters of all patients were collected and analyzed. The multivariate model based on regression analysis was performed to improve the diagnostic efficacy for discriminating papillary gastric adenocarcinomas preoperatively. The diagnostic performance of the established nomogram was evaluated by receiver operating characteristic curve analysis.
Results
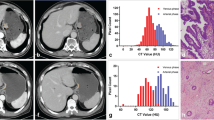
The distribution of age, carcinoembryonic antigen, differentiation degree, neural invasion, human epidermal growth factor receptor 2 overexpression, P53 mutation status, 4 CT morphological characteristics, and 10 CT valued-related parameters differed significantly between papillary gastric adenocarcinoma and tubular adenocarcinoma groups (all p < 0.05). The established multivariate model based on clinical information and CT findings for discriminating papillary gastric adenocarcinomas preoperatively achieved the area under the curve of 0.920.
Conclusion
There existed differences in clinicopathological features and CT findings between papillary gastric adenocarcinomas and tubular adenocarcinomas. The combination of demographic data, tumor markers, CT morphological characteristics, and CT value-related parameters could discriminate papillary gastric adenocarcinomas preoperatively with satisfactory diagnostic efficiency.
Graphical abstract








Similar content being viewed by others
Data availability
Data are available upon request to the corresponding author.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2020) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [published correction appears in CA Cancer J Clin 70(4):313]. CA Cancer J Clin 68(6):394-424.
Fukayama M, Rugge M, Washington MK (2019) Tumours of the stomach. In: WHO Classification of Tumours Editorial Board. Digestive system tumours WHO classification of tumours. 5th ed. Lyon: IARC. pp.59–110.
Kwon CH, Kim YK, Lee S, et al (2018) Gastric poorly cohesive carcinoma: a correlative study of mutational signatures and prognostic significance based on histopathological subtypes. Histopathology 72(4):556-568.
Nakamura K, Eto K, Iwagami S, et al (2022) Clinicopathological characteristics and prognosis of poorly cohesive cell subtype of gastric cancer. Int J Clin Oncol 27(3):512-519.
Tang CT, Chen Y, Zeng C (2020) Prognostic analysis of gastric signet ring cell carcinoma and mucinous carcinoma: a propensity score-matched study and competing risk analysis. Aging (Albany NY) 12(21):22059-22077.
Yu H, Fang C, Chen L, et al (2017) Worse Prognosis in Papillary, Compared to Tubular, Early Gastric Carcinoma. J Cancer 8(1):117-123.
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14(2):101–112.
Uesugi N, Sugai T, Sugimoto R, et al (2017) Clinicopathological and molecular stability and methylation analyses of gastric papillary adenocarcinoma. Pathology 49(6):596-603.
Wang XY, Yan J, Wu J, Zhang YF, Zhang GX (2019) A Comparison by Meta-Analysis of Papillary Early Gastric Carcinoma to Its Tubular Counterpart for the Risk of Lymph Node Metastasis and Submucosal Invasion. J Clin Gastroenterol 53(1):e19-e24.
Shin SY, Kim JH, Kook MC, et al (2021) Clinicopathologic Features of Submucosal Papillary Gastric Cancer Differ from Those of Other Differentiated-Type Histologies. Gut Liver 15(1):44-52.
Wang X, Deng J, Liang H (2021) Well differentiated carcinoma with a poor prognosis: a retrospective analysis of papillary gastric adenocarcinoma. Surg Today 51(8):1387-1396.
Cheng Y, Du M, Zhou X, et al (2021) High-grade Papillary Early Gastric Carcinoma With High Risk for Lymph Node Metastasis and Poor Prognosis: A Clinicopathologic Study of 96 Cases Among 1136 Consecutive Radical Gastrectomies. Am J Surg Pathol 45(12):1661-1668.
Lee IS, Park YS, Lee JH, et al (2013) Pathologic discordance of differentiation between endoscopic biopsy and postoperative specimen in mucosal gastric adenocarcinomas. Ann Surg Oncol 20(13):4231-4237.
Liu S, Liu S, Ji C, et al (2017) Application of CT texture analysis in predicting histopathological characteristics of gastric cancers. Eur Radiol 27(12):4951-4959.
Ravegnini G, Fosso B, Saverio VD, et al (2020) Gastric Adenocarcinomas and Signet-Ring Cell Carcinoma: Unraveling Gastric Cancer Complexity through Microbiome Analysis-Deepening Heterogeneity for a Personalized Therapy. Int J Mol Sci 21(24):9735.
Kim TS, Shin HC, Min BH, et al (2020) Favorable Long-Term Outcomes of Endoscopic Submucosal Dissection for Differentiated-Type-Predominant Early Gastric Cancer with Histological Heterogeneity. J Clin Med 9(4):1064.
Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P et al (2019) National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Gastric Cancer, Version 1.2019. Available at: https://www.nccn.org/professionals/physician_gls/PDF/gastric.pdf. Accessed 14 May.
You MW, Park S, Kang HJ, Lee DH (2020) Radiologic serosal invasion sign as a new criterion of T4a gastric cancer on computed tomography: diagnostic performance and prognostic significance in patients with advanced gastric cancer. Abdom Radiol (NY) 45(10):2950-2959.
Kim JW, Shin SS, Heo SH, et al (2012) Diagnostic performance of 64-section CT using CT gastrography in preoperative T staging of gastric cancer according to 7th edition of AJCC cancer staging manual. Eur Radiol 22(3):654–662.
Liu S, Qiao X, Xu M, Ji C, Li L, Zhou Z (2021) Development and Validation of Multivariate Models Integrating Preoperative Clinicopathological Parameters and Radiographic Findings Based on Late Arterial Phase CT Images for Predicting Lymph Node Metastasis in Gastric Cancer. Acad Radiol 28 Suppl 1:S167-S178.
Li R, Li J, Wang X, Liang P, Gao J (2018) Detection of gastric cancer and its histological type based on iodine concentration in spectral CT. Cancer Imaging 18(1):42.
Liu S, Qiao X, Ji C, et al (2021) Gastric poorly cohesive carcinoma: differentiation from tubular adenocarcinoma using nomograms based on CT findings in the 40 s late arterial phase. Eur Radiol 31(8):5768-5778.
Tsurumaru D, Miyasaka M, Muraki T, et al (2017) Histopathologic diversity of gastric cancers: Relationship between enhancement pattern on dynamic contrast-enhanced CT and histological type. Eur J Radiol 97:90-95.
Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y (2014) Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer 17(1):26-33.
Kambara Y, Miyake H, Nagai H, et al (2020) CA19–9 is a significant prognostic marker of patients with stage III gastric cancer. Eur J Surg Oncol 46(10 Pt A):1918–1924.
Kim SH, Lee JM, Han JK, et al (2005) Effect of adjusted positioning on gastric distention and fluid distribution during CT gastrography. AJR Am J Roentgenol 185(5):1180-1184.
Abrahao-Machado LF, Scapulatempo-Neto C (2016) HER2 testing in gastric cancer: An update. World J Gastroenterol 22(19):4619-4625.
Li N, Deng W, Ma J, et al (2015) Prognostic evaluation of Nanog, Oct4, Sox2, PCNA, Ki67 and E-cadherin expression in gastric cancer. Med Oncol 32(1):433.
Huang Q, Fang C, Shi J, et al (2015) Differences in Clinicopathology of Early Gastric Carcinoma between Proximal and Distal Location in 438 Chinese Patients. Sci Rep 5:13439.
Feng F, Tian Y, Xu G, et al (2017) Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer 17(1):737.
Koo DH, Ryu MH, Lee MY, et al (2021) Trends in Chemotherapy Patterns and Survival of Patients with Advanced Gastric Cancer over a 16-Year Period: Impact of Anti-HER2-Targeted Agent in the Real-World Setting. Cancer Res Treat 53(2):436-444.
Meric-Bernstam F, Johnson AM, Dumbrava EEI, et al (2019) Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin Cancer Res 25(7):2033-2041.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Institutional review board approval was obtained.
Informed consent
Written informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, M., Liu, S., Qiao, X. et al. Clinicopathological features and CT findings of papillary gastric adenocarcinoma. Abdom Radiol 47, 3698–3711 (2022). https://doi.org/10.1007/s00261-022-03635-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03635-w