Abstract
Purpose
To evaluate role of perfluorobutane in guiding microwave ablation of HCC and to compare treatment response at 3 h of ablation using contrast-enhanced US (CEUS) with Sonazoid with 1-month follow-up contrast-enhanced CT/MRI.
Methods
This was a single center prospective study and consecutive patients planned for microwave ablation of HCC from October to November 2021 were enrolled. Pre-procedure CEUS were performed using Sonazoid in both vascular and Kupffer phase and number of Kupffer defects compared with gray scale US. Precise needle placement of microwave applicator was done in the Kupffer phase. 3 hours post ablation CEUS was repeated to evaluate response assessment using Liver Imaging Reporting and Data System Treatment Response criteria (LR TR). One-month follow-up imaging was done using multiphasic CECT/dynamic CEMRI and comparison was done with post procedure CEUS.Please confirm if the author names are presented accurately and in the correct sequence (given name, middle name/initial, family name). Author 5 Given name: [Manoj Kumar] Last name [Sharma]. Author 6 Given name: [Shiv Kumar] Last name [Sarin]. Also, kindly confirm the details in the metadata are correct. all the names and affiliations are correct
Results
A total of 26 patients (24 males and 2 females, mean age 61.38 ± 9.76 years) having 40 lesions, of mean tumor diameter 21.4 ± 7.7 mm, underwent CEUS and ablation. Most common etiology for cirrhosis was viral hepatitis, followed by non-alcoholic steatohepatitis (NASH). Four (10%) additional lesions (which were seen on pre-procedure imaging) were detected in Kupffer phase over gray scale US. All lesions showed complete response in the immediate post procedure CEUS. Technique efficacy at 1-month was 95% according to the LR TR criteria.Please check the edit made in article title and amend if necessary.The edit is correct and appropriatePlease check and confirm that the authors and their respective affiliations have been correctly identified and amend if necessary.All the names and their respective affiliations are correct
Conclusion
CEUS with Sonazoid is an excellent modality for precise needle placement for ablation due to stable nature and excellent lesion visibility of Kupffer phase.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer related mortality worldwide [1]. Ultrasound (US) with or without alpha-fetoprotein (AFP) has been considered a standard technique of surveillance in high-risk population [2]. Attempts have been made to increase the efficacy of surveillance with introduction of US contrast agents as well as hepatobiliary phase of liver specific MRI contrast agents [2, 3]. Recent advances in diagnostic techniques, multimodality treatment, and post treatment surveillance have contributed to significant improvement in the diagnosis and prognosis of this disease [4, 5]. The importance of early detection and diagnosis of HCC lies in the complete treatment of small lesions via various surgical and percutaneous ablation techniques [4]. In percutaneous ablation techniques, radiofrequency ablation (RFA) has been the most widely used and studied modality, with newer techniques like microwave ablation (MWA), cryoablation etc. showing similar results.
Contrast-enhanced ultrasound (CEUS) can be a viable addition to gray scale US for surveillance as only the contrast agent need be added to the already established program of B-mode US [2]. However, pure blood pool contrast agents have limited utility as they act only during the vascular phase leading to limited window of examination [2, 6]. The recent advent of a US contrast agent Sonazoid (perfluorobutane) has ushered in a new era for surveillance, diagnosis, staging, and treatment planning of HCC [3,4,5, 7]. In addition to the vascular phase showing the microvasculature of the lesion, it also has a stable Kupffer phase which lasts from 10 to about 120 min. As hepatocellular carcinoma does not contain kupffer cells, the lesions appear as defects in the Kupffer phase in the background of a hyperechoic normal liver, whereas benign lesions contain Kupffer cells and will appear isoechoic to the background liver parenchyma [3, 7]. This stable phase can be used for screening, confirmation of diagnosis as well as guiding ablation of the tumor [2, 7,8,9,10]. Defect reperfusion imaging can also be used for confirmation of diagnosis [11].
Percutaneous ablation plays a key role in the treatment of early-stage HCC [12]. However, targeting of lesions under US alone may be misleading as there may be an enhancing component which is not seen on plain ultrasound. Sonazoid enhanced US has the advantage of vascular phase enhancement characteristics as well as a stable Kupffer phase which enables adequate targeting of lesions for ablation. There is limited availability of Sonazoid, leading to paucity of data on its efficacy. The purpose of this study is to evaluate the role of perfluorobutane in guiding microwave ablation of HCC and to compare treatment response at three hours post procedure using Sonazoid enhanced US with routine follow-up multiphasic CECT/Dynamic CEMRI at 1-month post procedure.
Methods
This was a single center prospective study with ethical approval taken from the Institute Ethical Committee (IEC) for the use of perfluorobutane-enhanced (Sonazoid; GE. Healthcare, Oslo, Norway) US for lesion detection, guidance and response assessment of HCC. A written informed consent was obtained from all patients for administration of Sonazoid pre and post procedure, and the risks and benefits of Sonazoid as well as ablation procedure explained. Informed consent was also taken from the patients for use of their clinical data for the purpose of this study. The protocol of the study was registered at https://ClinicalTrials.gov (NCT05068076).
Patient population
Consecutive patients planned for microwave ablation of HCC from October to November 2021 were enrolled in this study. Diagnosis of HCC was made in the pre-procedure imaging (CECT, CEMRI, or CEUS) according to the AASLD guidelines and Liver Imaging Reporting and Data System, using imaging for typical lesions (LR-4 and LR-5) [13]. Adult patients (18–80 years) with HCC, within the Milan Criteria with functional status at Child–Pugh A or B, who refused surgical resection were selected for microwave ablation. Lesions falling outside the Milan Criteria (size > 5 cm) were not included as efficacy of any ablation technique drastically reduces after lesion size crosses beyond 5 cm. Ill-defined lesions which were not well detected on B-mode US were also included. Exclusion criteria for ablation included severe coagulopathy (platelets < 50,000/ml, prothrombin time ratio < 50%), ongoing anticoagulants that could not be stopped, presence of vascular invasion and extrahepatic metastases at pre-procedure imaging study, poor performance status, larger lesions (more than 5 cm) requiring combined transarterial chemoembolization (TACE) and MWA, obstructive jaundice or any other concurrent malignancy. Patients with missing clinical or follow-up data were also excluded (Fig. 1).
Imaging
All pre-procedure imaging, whether multiphasic CECT, dynamic CEMRI or CEUS (in patients with renal dysfunction), was reviewed for the number, size and location of lesions, and their imaging characteristics. Clinical data of the patients were recorded and patients planned for microwave ablation.
All US examinations were done on an Aplio 500 US machine (Canon Medical Systems, Tokyo, Japan) using a 6C1 convex probe. Initial gray scale US examination of the whole liver was performed to look for the number of lesions identifiable on B-mode. This was followed by dual view imaging using a preset of CEUS with a mechanical index of 0.18 to 0.2. Image acquisition was done in the video format for the duration of the vascular phase followed by still image acquisition in the Kupffer phase from 15 to 20 min by a radiologist with 4 years of experience. These were then reviewed by another radiologist with 15 years of experience and inter-observer agreement calculated. Ablation was done in the Kupffer phase. This was followed by another CEUS three hours post procedure using the same format to assess therapeutic response. Images were evaluated in the arterial, venous, and delayed phases to look for arterial enhancement or washout. Ablation was considered successful if the ablation margins were more than 5 mm.
Routine follow-up imaging was advised 1 month after the procedure and included contrast-enhanced CT, MRI, or US. Post procedure CEUS was compared to this 1 month imaging to evaluate its usefulness in response assessment of HCC to ablation.
Contrast agent
Sonazoid consists of perfluorobutane gas microbubbles in a lipid shell with a diameter of 2–3 µm. It has a characteristic property of being retained by reticuloendothelial cells, particularly the Kupffer cells in the liver, which makes it different from all the other second-generation US contrast agents [3, 5, 7]. This leads to two phases where US assessment of lesions can be done, which are.
Vascular Phase (10 s to 5 min)—Shows tumor vascularity (similar to other contrast agents).
-
Arterial phase- 25–45 s
-
Venous phase- 45–70 s
-
Delayed phase- 3–5 min
Kupffer phase (10 min to 60 min)—Accumulation in reticuloendothelial cells.
-
Liver appears hyperechoic
-
Lesions appear hypoechoic (called Kupffer defects) as hepatocellular carcinomas do not contain Kupffer cells.
The drug is reconstituted with 2 ml distilled water. Standard dosage of contrast varies from 0.01 to 0.015 ml/kg [5]. 1 ml of the contrast agent was administered on table followed by flushing with 10 ml saline and CEUS performed in the vascular phase. Kupffer phase imaging was done at 15–20 min to locate all the Kupffer defects and comparison was made with B-mode US. Lesions were clearly demarcated in this phase which were hypoechoic compared to the surrounding parenchyma. Precise needle placement was done in the centre of the Kupffer defect and ablation was carried out. As Sonazoid is stable for 4–6 h after reconstitution, the same vial was used for the post procedure CEUS at 3 h.
Ablation
All ablation procedures were done on an intention-to-treat basis. The procedure was performed under conscious sedation and local anesthesia along with monitoring of heart rate, blood pressure, and saturation of oxygen during the procedure. Conscious sedation was achieved by initial IV injection of midazolam 1–2 mg, propofol 30 mg and fentanyl 50 μg. This was followed by boluses of propofol 30 mg and fentanyl 50 μg titrated according to sedation and pain. Ablation was performed either using 19 cm cooled-tip Solero microwave applicator (Angiodynamics, Marlborough, USA). Ablation margin of > 5 mm was considered adequate for ablation. Needle track ablation was done after all ablations.
Treatment outcome and follow-up
Patients were monitored in the hospital for 1–2 days to look for post ablation syndrome and any procedure-related complications.
Follow-up was scheduled at 4 weeks of ablation, consisting of clinical examination of the patient followed by contrast-enhanced CT/MRI scan of the abdomen and liver function tests. The imaging evaluation in post procedure CEUS and follow-up imaging was done using Liver Imaging Reporting and Data System treatment response (LR-TR) criteria (Table S1) [14, 15]. Treatment response at 3 h post ablation CEUS and 1-month follow-up imaging were compared. Technical success was defined as the ablation zone completely covering the tumor with an ablative margin of at least 5 mm in the immediate post procedure period and technique efficacy was defined as no residual disease at 1 month follow-up imaging (according to the image-guided tumor ablation: standardization of terminology and reporting criteria) [16]. Objective response rate was defined as a sum of complete and partial response rate.
Statistical analysis
Continuous variables were denoted as median with range or mean ± standard deviation and compared using independent t-test or the Mann–Whitney U-test. Categorical variables were expressed as proportions and compared using the Fisher’s exact probability test. κ test was used to compute inter-observer agreement. The technique efficacy was calculated using the McNemar test. All statistical analysis was performed using SPSS v.22.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 26 patients (24 males and 2 females) with 40 lesions underwent CEUS and ablation. The mean age of the study group was 61.38 ± 9.76 (range 38 to 82 years) and the mean tumor diameter was 21.4 ± 7.7 mm (range 9 to 37 mm). The most common etiology for cirrhosis in the study group was viral hepatitis, followed by non-alcoholic steatohepatitis (NASH). 65.4% patients belonged to Child class A and 57.7% patients belonged to very early stage of Barcelona Clinic Liver Cancer (BCLC) staging. One patient had associated renal failure and CEUS was used as a diagnostic imaging modality. On pre-procedure imaging, 36 lesions were LR 5 and the remaining 4 lesions were LR 4. Baseline characteristics, laboratory parameters, and tumor characteristics are shown in Table 1.
Please check the layout of Table 1 correct if necessary.all tables are correct
Pre-procedure CEUS
The total number of lesions seen on pre-procedural imaging were 40 in 26 patients, and all were planned for microwave ablation. However, on gray scale US, only 36/40 lesions could be identified. On pre-procedural CEUS, all 40 lesions could be seen as Kupffer defects. The mean lesion size on gray scale US was 21.1 ± 7.8 mm whereas on Kupffer phase it was 21.9 ± 7.4 mm (p = 0.65). Therefore, additional lesions identified by Sonazoid over B-mode US were 10% (4/40). There was good inter-observer variability between the two observers for arterial enhancement (ƙ = 0.817) and complete agreement for washout and Kupffer phase defects (ƙ = 1.000). Final observations were agreed upon by consensus (Table 2).
Post Procedure CEUS and follow-up imaging
All 40 lesions showed > 5 mm ablative margins on CEUS with no evidence of any arterial enhancement or washout. Thus all lesions were LR TR non-viable (100%) and all 26 patients showed complete response (100%). Eighteen patients (69.2%) underwent CECT as their 1 month follow-up imaging, 6 (23.1%) underwent CEMRI and the remaining 2 underwent CEUS (as one patient developed renal failure in the intervening period between the first imaging modality, and 1-month follow-up after ablation). At 1-month follow-up imaging, two lesions in two different patients showed subtle peripheral arterial enhancement with washout on venous phase. One of the patients underwent a Triphasic CECT and the other underwent multiphasic MRI. Both patients underwent re-ablation after review of imaging.
Thus, the technique efficacy as per definition was 95% (38/40) according to LR TR criteria (Table 3). No complications or mortality were seen either due to drug administration or due to ablation procedure. The objective response rate was 100%. Figures 2 and 3 show representative cases.
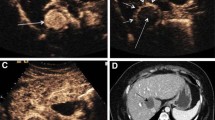
A 62-year-old-male presented with alcoholic cirrhosis with Child Pugh class A and very early stage of BCLC A, B Axial CT arterial and venous phase images show a single lesion in segment VI showing intense enhancement followed by washout. C Gray scale image showing a well-defined tumor (arrow) and corresponding CEUS image showing intense arterial enhancement. D Venous images of CEUS showing washout. E Delayed (15 min) image showing Kupffer defect (arrow) as compared to surrounding liver. F Lesion targeting in Kupffer phase with MWA needle (arrow) seen in situ. G Ablation of HCC. H Post procedure CEUS showing no enhancement seen in the ablated zone. I 1-month post procedure CT showing no abnormal arterial enhancement (complete response)
A 52-year-old-male presenting with HBV-related cirrhosis with Child class B and very early stage of BCLC with a single lesion. A, B Dual view CEUS images show an ill-defined lesion in segment VII on gray scale showing patchy arterial enhancement (arrow), followed by washout. C Kupffer phase (15 min) image showing hypoechoic area compared to the surrounding liver. D No abnormal enhancement seen in immediate post procedure CEUS with needle track (arrow) seen in the centre of the lesion. E, F Subtle peripheral arterial enhancement and washout (arrows) seen in 1-month follow-up CT
Discussion
This study was aimed at evaluating the role of Kupffer phase specific contrast agent in lesion detection, targeting for ablation, and response assessment after ablation. The mean age of the study group was 61.38 ± 9.76 years. Additional 10% lesions were detected on Kupffer phase imaging and all lesions were successfully targeted in the Kupffer phase. Response assessment and comparison was done between post procedure CEUS and 1 month follow-up imaging, resulting in a technique efficacy of 95% using LR TR criteria with an objective response rate of 100%.
CEUS with Sonazoid has been used in Japan, South Korea, Denmark, and Norway for diagnosis and surveillance and has been recently approved in China [2]. However, it has not yet been approved in other countries, so only limited studies have been done on its efficacy in diagnosis and guiding ablation. In a comparison study between Sonazoid and another second-generation contrast agent Sonovue for detection of focal liver lesions, it was concluded that the improvement in diagnostic accuracy was 0.30 in the Sonazoid group and 0.16 in the Sonovue group. In addition, the number of lesions detected by Sonazoid on whole liver scanning was significantly more than Sonovue (p = 0.024) [6]. Thus, it has a similar diagnostic accuracy as compared to other frequently used US contrast agents and is significantly better in whole liver scanning due to its Kupffer phase.
In a large prospective multicentre trial (SCAN), in which Sonazoid was used for surveillance of HCC, it was seen that of the 10 HCC confirmed in eight patients, eight were detected by gray scale US, one additional lesion be Sonazoid enhanced US and one lesion was not detected even on CEUS. Although the detection rate was not significantly improved by use of Sonazoid, the false referral rate was significantly reduced [2]. In a retrospective study, it was reported that CEUS with Sonazoid resulted in detection of an additional 14% of the lesions in the Kupffer phase which were not detected on B-mode US. Three lesions were not detected even on US [8]. In comparison, this study showed additional 10% lesions in the Kupffer phase over gray scale and no lesion was missed. Another retrospective study used perflutren lipid (Definity) as the contrast agent for lesion detection and reported 79% additional lesion detection over conventional US [17].
Few studies have used CEUS for guiding radiofrequency ablation of HCC. This study has used microwave as the ablation technique which has not been reported in any previous study. Also, average lesion size on Kupffer phase was more than on gray scale US in this study (although p value was not significant). This reinforces the importance of using Kupffer phase for needle puncture and guidance as ablation margins may not be accurate on gray scale imaging alone and may lead to undertreatment of the lesion. Numata et.al [8] successfully used Sonazoid enhanced US for guiding RFA in 93% of patients. CEUS was not used for response assessment in this study. Another study reported successful ablation in all 19 patients when RFA was performed under CEUS guidance, however, they used Sonovue as the contrast agent [18]. In the present study as well, microwave ablation was successfully used for guidance in all patients using Kupffer phase.
A number of studies have assessed therapeutic response in the immediate post ablation period using contrast-enhanced US. Adequate ablative margins on Sonazoid enhanced US were seen in 89.7% of the patients 3 h post ablation [9]. Another study reported 100% complete response rate using 30 min post procedure Sonovue enhanced US [18]. In a meta-analysis by Shi et al. [10], CEUS after RFA for response assessment had a 91% overall success rate, with subgroup analysis showing better success with Sonazoid than Sonovue (95% vs 87%). In the present study, technique efficacy rate was 95% using LR TR criteria which is similar to the previous studies.
The timing for post ablation CEUS was variable from 30 min to 3 h [9, 18]. A study was conducted to evaluate the best time for treatment response assessment after radiofrequency ablation [19]. It was concluded that the tumor boundaries were best evaluated during 15–22 h period, followed by 3–6 h. Thereafter the diagnostic accuracy decreased over 5 days. The authors performed CEUS 3 h post procedure as most of the bubbles dissipate during this time and because the same reconstituted contrast as pre-procedure CEUS was used to control cost.
Traditionally, 1-month follow-up contrast cross sectional imaging is done for evaluation of treatment response as reactive hyperemia has settled by then [19]. However, with improving US machines and contrast agents, especially Sonazoid, accurate response assessment can be done in the immediate post-operative period. There is change in the echogenicity of the tumor, tumor margin and surrounding ablative area over 5 days which can be assessed on CEUS [19]. Immediate post procedure in the present study showed complete response in all patients however residual disease was seen in two patients on follow-up imaging. This trend was also seen in the meta-analysis where success rate within 24 h evaluation was 94% which dropped to 91% at 1 month evaluation [10]. This can be because ablation induced hypovascular area can appear larger than true ablative margins in the immediate post-operative period in ill-defined lesions, due to devascularisation and reactive hyperemia in the already ill-defined margins of the lesions [9, 10, 19]. In addition, area of thermal injury is more than ablation. It was postulated that vessels may be injured or spasmed in the immediate post-op period which leads to non-enhancement of a larger area. Gradually over time, the spasm is released and periphery may show residual enhancement in follow-up imaging. Biopsy was also not done to confirm the histopathology thus creating a scope to miss combined histological entity occurring in cirrhotic background (Intrahepatic cholangiocarcinoma/ combined hepato-cholangiocarcinoma), which may be a contributory factor to presence of residual disease.
Sonazoid has a very good safety profile with minimal complications which may include mild symptoms like diarrhea, headache, proteinuria, rash, dry mouth etc. [5]. No Sonazoid or microwave-related complications were seen in this or any previous study establishing the excellent safety profile of the drug. Low mechanical index required for imaging in CEUS may sometimes limit its utility in deep lesions or obese patients where the acoustic window can become poor for examination [4, 5].
The strength of this study lies in its prospective nature with utilization of microwave ablation which has not yet been studied. Comparison was made with cross sectional imaging at 1 month follow-up and residual lesions were planned for re-intervention. Limitations of this study include operator dependence for US examinations, relatively small number of patients and no comparison group. Multicenter prospective studies/randomised controlled trials with a comparison group are required to further substantiate the findings of this study. These studies should also include biopsy of indeterminate or LR4 lesions prior to ablation to improve outcome efficacy and avoid residual lesions. In addition, predictive factors for residual disease can also be determined, and quantitative dynamic parameters of CEUS can be used for better characterisation of focal lesions.
In conclusion, CEUS with Sonazoid is an excellent modality for precise needle placement for guiding ablation due to the very stable nature and excellent lesion visibility of Kupffer phase, with a high technique efficacy rate and objective response rate. Kupffer phase can also help in detecting additional lesions which can be missed on simple gray scale US and CEUS using other contrast agents.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics. CA Cancer J Clin 2012. 2015;65:87-108.
Park JH, Park M-S, Lee SJ, et al. Contrast-enhanced US with Perfluorobutane for Hepatocellular Carcinoma Surveillance: A Multicenter Diagnostic Trial (SCAN). Radiology. 2019 Sep;292(3):638–46.
Kudo M. Diagnostic imaging of hepatocellular carcinoma: recent progress. Oncology. 2011;81 Suppl 1:73-85.
Minami Y, Kudo M. Contrast-enhanced ultrasonography with Sonazoid in hepatocellular carcinoma diagnosis. Hepatoma Res. 2020;6:46
Maruyama H, Sekimoto T, Yokosuka O. Role of contrast-enhanced ultrasonography with Sonazoid for hepatocellular carcinoma: evidence from a 10-year experience. J Gastroenterol. 2016 May;51(5):421–33
Zhai H-Y, Liang P, Yu J, et al. Comparison of Sonazoid and SonoVue in the Diagnosis of Focal Liver Lesions: A Preliminary Study. J ultrasound Med Off J Am Inst Ultrasound Med. 2019 Sep;38(9):2417–25
Kudo M, Hatanaka K, Maekawa K. Sonazoid-enhanced Ultrasound in the Diagnosis and Treatment of Hepatic Tumors. J Med Ultrasound. 2008;16(2):130-139
Numata K, Morimoto M, Ogura T, et al. Ablation therapy guided by contrast-enhanced sonography with Sonazoid for hepatocellular carcinoma lesions not detected by conventional sonography. J ultrasound Med Off J Am Inst Ultrasound Med. 2008 Mar;27(3):395–406
Nishigaki Y, Hayashi H, Tomita E, et al. Usefulness of contrast-enhanced ultrasonography using Sonazoid for the assessment of therapeutic response to percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2015 Apr;45(4):432-40.
Shi W, He Y, Ding W, et al. Contrast-enhanced ultrasonography used for post-treatment responses evaluation of radiofrequency ablations for hepatocellular carcinoma: a meta-analysis. Br J Radiol. 2016 Aug;89(1064):20150973
Kudo M. Defect Reperfusion Imaging with Sonazoid®: A Breakthrough in Hepatocellular Carcinoma. Liver cancer. 2016 Feb;5(1):1–7.
Bruix J, Sherman M. Practice guidelines committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–36.
Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53(3):1020–1022.
Gupta P, Bansal A, Das GC, et al. Diagnostic accuracy of Liver Imaging Reporting and Data System locoregional treatment response criteria: a systematic review and meta-analysis. Eur Radiol. 2021 Oct;31(10):7725-7733.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30(1):52–60.
Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273(1):241-260.
Chan AKW, Hegarty C, Klass D, et al. The Role of Contrast-enhanced Ultrasound in Guiding Radiofrequency Ablation of Hepatocellular Carcinoma: A Retrospective Study. J l’Association Can des Radiol. 2015 May;66(2):171–8.
Rajesh S, Mukund A, Arora A, Jain D, Sarin SK. Contrast-enhanced US-guided radiofrequency ablation of hepatocellular carcinoma. J Vasc Interv Radiol. 2013 Aug;24(8):1235–40
Zhou P, Kudo M, Minami Y, et al. What is the best time to evaluate treatment response after radiofrequency ablation of hepatocellular carcinoma using contrast-enhanced sonography? Oncology. 2007;72 Suppl 1:92-7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mukund, A., Bansal, A., Patidar, Y. et al. Role of contrast-enhanced ultrasound with Perfluorobutane in lesion detection, guidance for microwave ablation, and response assessment of hepatocellular carcinoma. Abdom Radiol 47, 3459–3467 (2022). https://doi.org/10.1007/s00261-022-03609-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03609-y