Abstract
Purpose
Adequate TNM-staging is important to determine prognosis and treatment planning of duodenal adenocarcinoma. Although current guidelines advise contrast-enhanced CT (CECT) for staging of duodenal adenocarcinoma, literature about diagnostic tests is sparse.
Methods
In this retrospective single-center cohort study, we analyzed the real life performance of routine CECT for TNM-staging and the assessment of resectability of duodenal adenocarcinoma. Intraoperative findings and pathological staging served as reference standard for resectability, T-, and N-staging. Biopsies, 18FDG-PET-CT, and follow-up were used as the reference standard for M-staging.
Results
Fifty-two consecutive patients with duodenal adenocarcinoma were included, 26 patients underwent resection. Half of the tumors were isodense to normal duodenum on CECT. The tumor was initially missed in 7/52 patients (13%) on CECT. The correct T-stage was assigned with CECT in 14/26 patients (54%), N-stage in 11/26 (42%), and the M-stage in 42/52 (81%). T-stage was underestimated in (27%). The sensitivity for detecting lymph node metastases was only 24%, specificity was 78%. Seventeen percent of patients had indeterminate liver or lung lesions on CECT. Surgery with curative intent was started in 32 patients, but six patients (19%) could not be resected due to unexpected local invasion or metastases.
Conclusion
Radiologists and clinicians have to be aware that routine CECT is insufficient for staging and determining resectability in patients with duodenal adenocarcinoma. CECT underestimates T-stage and N-stage, and M-stage is often unclear, resulting in futile surgery in 19% of patients. Alternative strategies are required to improve staging of duodenal adenocarcinoma. We propose to combine multiphase hypotonic duodenography CT with MRI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Duodenal adenocarcinoma (DA) is a rare malignancy; it represents less than 1% of all gastrointestinal tract tumors [1,2,3]. However, the incidence is increasing: in the United States of America (USA) the incidence of small intestine tumors has more than doubled from 4,700/year to 11,110/year in the past 20 years. However, data for DA specifically are not available for the USA [4, 5]. For the Netherlands, these data are available: with a more than fourfold increase of the incidence of DA from 46 patients in 2000 to 192 patients in 2020 [1, 2].
While DA represent over 50% of small bowel adenocarcinoma, they differ from other small bowel tumors: the resection rate of 41–57% for DA is substantially lower than the 83–95% resection rate for jejunal and ileal cancer, and the median overall survival (OS) of 13–40 months for DA compares unfavorable to 19–63 months for jejunal and ileal cancer [2, 3, 6, 7]. Because DA is a rare disease, the majority of publications that are available discuss all small bowel tumors as one group or include DA in the analysis of periampullary tumors [8,9,10,11,12].
Surgical resection is the only potentially curative treatment for DA. Without resection, 5-year survival is only 1%, after resection this increases to 46% [13]. The presence of lymph node (LN) metastases in the resection specimen reduces 5-year survival to 21% compared to 65% in patients without LN metastases [13]. Approximately one-third (34–38%) of patients with DA have distant metastasis at the time of diagnosis [2, 6], generally leading to palliative treatment.
DA [14]. New treatment strategies may include neoadjuvant treatment, similar to esophageal and rectal cancer [13, 15, 16], or local treatment of oligometastatic disease, similar to strategies in colorectal cancer [17, 18]. Adequate staging to select patients who could benefit from these treatment strategies is essential.
Two guidelines are currently available for small bowel adenocarcinoma: the 2017 French intergroup clinical practice guidelines [19] and the 2020 NCCN guideline version 2.2020 [20]. Recommendations for the diagnostic work-up in the French guideline are short and based on expert opinion only; they advocate CECT for primary staging. The newer NCCN guidelines on small bowel adenocarcinoma provide more detailed guidelines for the diagnostic work-up of DA. CECT is recommended as primary staging modality. EUS, MRI, and 18FDG-PET-CT are recommended as problem-solving tools if CT is equivocal. Recommendations are mostly based on studies on small bowel carcinoma. While both guidelines advise CECT as the primary staging modality, none of the two provides data about the accuracy of CECT for DA. In this study, we analyze the performance of CECT in the diagnostic work-up for patients with DA at a tertiary referral hospital.
Materials and methods
This is a retrospective single-center cohort study to determine the real life performance of routine CECT for TNM-staging and resectability assessment of duodenal adenocarcinoma. The institutional review board approved the study and waived informed consent.
Patients
We identified all patients with DA at our institution between January 1st of 2000 and June 1st of 2020. To identify patients we searched the patient files using 11 synonyms for ‘duodenal neoplasm’ in Dutch and English. Patients with histopathologically proven DA and a CECT scan available were included. Patients were excluded if they had opted out for use of their data for research, if they had a tumor of the pancreas, bile duct, or ampulla of Vater, if the histologic subtype was not adenocarcinoma, if simultaneously another malignancy was present, or if time between CECT and surgery was over 8 weeks. Patients primarily diagnosed at our institution as well as referred patients were included.
Data acquisition
Patient records were reviewed to obtain clinical data, including: age, sex, diagnostic modality the tumor was first detected with, CT parameters (contrast phases, use of oral contrast agent, slice thickness, scan year), and use of problem-solving tools (18FDG-PET-CT, MRI, and EUS). Patients who underwent surgery had the following variables collected: curative or palliative intent of surgery; if a resection was performed or not; and reason why resection was not performed (unexpected distant metastases or local invasion). Pathological TNM stage was retrieved from the pathology reports (TNM 8th edition, small Intestine: Adenocarcinoma, of the American Joint Committee on Cancer staging [21]).
Imaging analysis
A radiologist with 20 years of experience in abdominal radiology retrospectively evaluated all CECT scans, blinded for the clinical data. All available contrast-phases were used for the evaluation of the CT scans. Evaluation included tumor location (duodenal segment), contrast-enhancement of the tumor in the portal-venous phase (PVP) compared to the healthy duodenal wall, and resectability of the tumor.
Resectability was classified as; resectable, borderline, and irresectable and was based on vessel contact with coeliac trunk, superior mesenteric artery (SMA), common hepatic artery (CHA), superior mesenteric vein (SMV), portal vein, inferior caval vein, and jejunal arteries and veins. Vessel contact was categorized as ≤ 90, 90–180, 180–270, and > 270 °. Criteria for resectability for pancreatic cancer of the Dutch Pancreatic Cancer Group (DPCG) [22] were used as a guideline, but because these criteria are not designed for DA and therefore suboptimal, final classification (resectable, borderline resectable, or irresectable) was according to expert opinion. The criteria from the DPCG are; resectable: no arterial contact and venous contact ≤ 90 °; borderline resectable: arterial contact ≤ 90 ° and/or venous contact 90°–270° and no occlusion; locally advanced indicated to be irresectable: arterial contact > 90° and/or venous contact > 270° or occlusion. Finally, clinical TNM stage (again 8th edition, small Intestine: Adenocarcinoma) was assessed on CECT. T1 and T2 were combined, because distinction between invasion of lamina propria, submucosa, and muscularis propria is not possible on CECT. Tumors that were not visible were classified as Tx. LNs with a short axis of ≥ 10 mm on axial orientation were considered malignant. We combined N1 and N2 to get a binary outcome that enables us to calculate sensitivity and specificity of CECT for the detection of LN metastases.
Only resected patients were included in the T- and N-stage analysis. The pathological T- and N-stage served as reference standard for comparison to the clinical T- and N-stage on CECT. All patients were included in the M-stage analysis, for which histopathological confirmation by biopsy, clinical confirmation with 18FDG-PET-CT, or growth during follow-up was used as reference standard. Indeterminate lesions on CECT (Mx), which could not be verified by the reference standard, remained Mx as final M-stage. After the radiologist analyzed all scans, a surgeon with 24 years of experience with pancreatic and hepato-biliary surgery evaluated resectability on CECT similarly as the radiologist (expert opinion, with the DPCG criteria as guideline). The surgeon was blinded for the given treatment, patient outcome and the radiologists’ assessment of resectability, but was informed about all other features evaluated by the radiologist.
Statistical analysis
Data were analyzed using the IBM SPSS Statistics 25 software package (IBM Corporation). Continuous variables were summarized with standard descriptive statistics including mean, standard deviation, median, and range. Categorical variables were summarized with frequencies. A p-value below 0.05 was considered statistically significant. Accuracy of LN staging was calculated using cross-tabulations.
Results
Patient characteristics and diagnosis
Eighty-four patients with suspected DA were identified of which 52 patients met the inclusion criteria, 32 patients were excluded because the final diagnosis was not DA (n = 18), there was no CECT available (n = 4), the time between CECT and surgery was more than 8 weeks (n = 6), there was a simultaneous other malignancy (n = 3), or there was no histopathological prove (n = 1). None of the eligible patients had opted out for use of their data for research. Table 1 summarizes patient characteristics. In most patients endoscopy was the modality on which the diagnosis DA was first suspected, followed by CECT. In 13 patients, the diagnosis was initially missed at endoscopy (6/52, 12%), CECT (4/52, 8%), or both (3/52, 6%). The median delay between missed diagnosis and final diagnosis was 12 weeks (1–49 weeks) for endoscopy and five weeks (1–103 weeks) for CECT.
CECT parameters
Scan protocols were variable because the study covers a long time range and 75% (39/52) of patients were referred with a CECT scan from other non-academic hospitals. Additionally, in clinical practice most CECT scans are performed because of a specific symptoms, not targeted at DA specifically, usually resulting in a CECT with only a PVP. In our dataset in 14 cases CECT was not targeted at staging DA specifically, but CECT was performed because of analyses of symptoms like weight loss. In the other 38 cases it was known that a duodenal tumor was present or there was a high suspicion. Thirteen CECT scans dated before 2015 (2000–2004 n = 1; 2005–2009 n = 3; 2010–2014 n = 9) and 39 scans dated after 2015. Table 2 compares parameters between scans dated before and after 2015. Contrast type, concentration, volume, flowrate, and delay were not available in the majority of cases due to the retrospective nature of the study.
Tumor characteristics on CECT
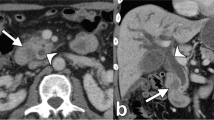
Contrast-enhancement of the tumor compared to normal duodenum on the PVP was classified as; hyperdense, isodense, hypodense, or mixed density. Fifty percent was isodense (26/52) and 10% was not visible on CECT (5/52, Figs. 1 and 2). Tumors mostly involved D2, the descending part (21/52, 40%), with nine of these tumors located in multiple segments including D2.
Resectability
Involvement of arteries and/or veins that are included in the DPCG criteria for resectability of pancreatic cancer (SMA, coeliac trunk, CHA, SMV, and portal vein) was seen in 11 patients (11/52, 21%). Vascular involvement of jejunal arteries and/or veins, which are not included in the DPCG criteria but, depending on the extent, can render a patient inoperable, occurred in 15 patients (15/52, 29%). Involvement of the inferior caval vein, also not included in the DPCG criteria, occurred in three patients. Examples are shown in Fig. 3. Seventy-three percent (11/15) of the tumors with vascular involvement of the jejunal arteries and veins involved the third or fourth duodenal segment.
Examples of vascular involvement (indicated with white arrows). a Coronal view of a tumor in D3 with involvement of jejunal arteries and veins in the mesenteric root. b Axial view of the same tumor as a. c Axial view of a tumor in D3 with abutment of inferior caval vein. d Sagittal view of the same tumor as c
Thirty-two patients underwent surgery with a curative intent, of which 26 were resected. Six patients (19%) could not be resected because of unexpected local invasion (4/6) and/or unexpected metastases (3/6). Palliative bypass surgery (gastroenterostomy) was performed in these six patients. On second look in three of these six patients the surgical findings that hampered resection could not be reproduced on preoperative CT scan and in three patients there were in retrospect some indications visible on preoperative CT scan (indeterminate liver lesions or slight vessel induration). Twenty patients received primary palliative treatment, because of metastases (n = 7), locally advanced disease (n = 2), both (n = 2), or insufficient performance status (n = 9). Palliative bypass surgery was performed in five of these 20 patients. Data are displayed in Fig. 4.
Stacked bar chart representing all patients (n = 52). In the left bar, all patients that underwent surgery with a curative intent (n = 32) and in the right bar patients with primary palliative treatment (n = 20) are displayed. If patients were resected and the reasons patients were not resected are displayed within the bars
Of the patients that underwent surgery with a curative intent (n = 32) the radiologist and surgeon, that individually evaluated resectability, agreed in 88% (n = 28) and disagreed in four cases (1 × radiologist irresectable vs surgeon resectable; 2 × radiologist borderline vs surgeon resectable; 1 × radiologist resectable vs surgeon borderline). Most disagreements (n = 3) were due to a difference in opinion whether the jejunal vein or artery contact would technically allow a resection or not. In one case there was some induration around the AMS, that the radiologist thought not to be tumor contact but inflammation and the surgeon thought it was tumor contact (at surgery there was involvement of the AMS and of several surrounding organs). Of both experts five patients classified as resectable and one patient as borderline, could unexpectedly not be resected during surgery. One patient classified as irresectable by the radiologist was resected. Data are displayed in Fig. 5.
TNM-staging on CECT
T- and N-stage on CECT of resected patients (n = 26) was correlated to histopathology. The T-stage was correctly classified with CECT in 14 patients (54%), underestimated in seven (27%), overestimated in three (12%), and could not be determined in two patients (8%) (Table 3). In patients with an incorrect T-stage the tumor was more often isodense compared to a correct T-stage (8/10 vs 7/14, p = 0.21).
Of the resected patients on CECT, there were four patients with N0, eight patients with N1, and 14 patients with N2 and at histopathology there were nine patients with N0, eight patients with N1, and nine patients with N2. The presence (N1 or N2) or absence (N0) of LN metastases was correctly predicted in 11 patients (42%), false positive in two (8%), and false negative in 13 (50%). Sensitivity and specificity for detecting LN metastases on CECT was 24% and 78%, respectively (Table 4).
The results of M-staging for all 52 patients are provided in Table 5. Ten patients were suspected of having metastases: in the liver (n = 7), peritoneal (n = 2), or at multiple sites (n = 1). These were all confirmed by histopathology, 18FDG-PET-CT, or follow-up. Additionally, in one patient peritoneal metastases were not visible on CECT. Nine patients showed indeterminate lesions (Mx) on CECT: in the liver (n = 7), the lung (n = 1), or both (n = 1). In one patient, these lesions were confirmed to be metastases; five patients had no metastases according to the reference standard; and in three patients it remained indeterminate. The M-stage was correct on CECT in 42 patients (81%), underestimated in one patient (2%) and unclear in nine patients (17%).
Problem solving tools (18FDG-PET-CT, MRI, and EUS)
In 23 patients MRI, in 16 patients 18FDG-PET-CT, and in five patients EUS was performed. In seven patients (7/23, 30%), MRI provided useful additional information: detection of primary tumor (n = 1), confirmation of (suspected) liver metastases (n = 3), detection of liver metastases (n = 1), and better assessment of tumor extension (n = 2). In two patients ,the MRI was false positive for liver metastases. In eight patients (8/16, 50%), 18FDG-PET-CT provided useful additional information: detection of primary tumor (n = 2), confirmation of (suspected) liver metastases (n = 5), and confirmations that suspect liver lesions on CECT were not metastases (n = 1). EUS was mainly used to obtain histopathology (n = 4) and in one patient the tumor was detected during EUS.
Discussion
Our study shows that CECT, which is recommended in clinical guidelines, is insufficient for staging and prediction of resectability of duodenal adenocarcinoma. TNM-staging of DA with CECT was correct for T-stage in 54%, N-stage in 42%, and M-stage in 81%. T-stage was frequently underestimated (27%). Sensitivity of CECT for detecting LN metastases was only 24% and specificity was 78%. M-stage with CECT was correct in 81%, but a substantial number of patients (9/52, 17%) had indeterminate liver or lung lesions.
Nine-teen percent (6/32) of patients, who underwent surgery with curative intent, could not be resected due to unexpected local vessel invasion and/or metastases. This is in line with a study on diagnostic laparoscopy in patients with radiologically resectable DA on CECT, where 16% (6/38) was irresectable due to vascular invasion or metastases [23]. In our study the surgeon was more optimistic about resectability and was more frequently correct in resectability prediction than the radiologist. Involvement of jejunal veins and/or arteries was common (15/52), especially in tumors involving the third and fourth duodenal segment. This is in accordance with a study by Stell et al. that also found that lesions of the third and fourth segment duodenum are less often resectable due to invasion of the small bowel mesentery [24]. A lesson learned from our study is that besides vascular contact of the main surrounding arteries and veins that are included in the DPCG criteria, the first jejunal artery and vein are relevant to determine resectability, especially in tumors of the third and fourth segment of the duodenum. In case of near complete encasement of the first jejunal artery and vein, a tumor could be considered as irresectable. However, if after peroperative clamping of the first jejunal artery a limited segment of bowel is affected we consider this as resectable.
In our study, tumor enhancement on CECT was isodense to normal duodenum in 50% of the patients. No tumor could be visualized on CECT in 10%. These findings are comparable with a study analyzing presurgical imaging for resectable periampullary cancer [25]. The inability to visualize the tumor on CECT was not related to size or location. The low performance of CECT to determine T-stage might be explained by the high number of isodense tumors, which were more likely to be staged incorrectly, although this was not statistically significant. Imaging techniques aimed at visualizing the duodenum and tissue characteristics could potentially improve tumor detection, characterization, and staging. For example hypotonic CT with negative or neutral oral contrast agent has shown good results for T-staging of gastric cancer [26, 27]; and hypotonic MRI duodenography with water ingestion resulted in good visualization of the duodenal lumen and wall in healthy volunteers [28]. Hypotonic duodenography could also be used to asses if a tumor is annular or has a polypoid morphology. In a prospective study comparing MRI-enterography to CT-enterography in 150 patients with suspected small bowel disease, MR was more accurate for neoplastic diseases [29]. However, no patients with DA were included in any of these studies. Dual Energy CT is an emerging technique with promising results in other abdominal malignancies. For example the assessment of early versus advanced stage gastric cancer, and the distinction of histological origin of carcinomas in the ampullary region [30, 31].
In our study in some patients 18FDG-PET-CT (8/16) and MRI (7/23) provided additional useful information, but MRI also falsely indicated liver metastases in two patients. For the detection of LN metastases, USPIO-enhanced MRI (ultra-small superparamagnetic iron oxide) has proven to be valuable in solid tumors, like prostate and breast cancer [32, 33], but no data are available for small bowel cancers. Diffusion-weighted MR imaging (DWI) has shown to be useful for detecting distant metastases in colorectal cancer [34], but again no data for DA are available. While 18FDG-PET-CT may be useful for diagnosing and staging of DA, only a small subset of patients in our study underwent 18FDG-PET-CT and literature about 18FDG-PET-CT for DA is limited to case series and case reports [35, 36]. 68 Ga-FAPI-PET-CT is a new technique that has shown promising results in the detection of the primary tumor, lymph node metastases, and distant metastases in gastrointestinal cancers, with a higher sensitivity compared to conventional 18FDG-PET-CT [37].
Duodenal adenocarcinoma is a rare tumor, but literature shows that DA is different in behavior and outcome compared to periampullary or small bowel adenocarcinomas [6, 38,39,40]. Studies focusing on diagnostics of DA are rare and the number of patients with DA included in diagnostic studies is low. To our knowledge, this study is the only study within the past decades that systematically analyzed the performance of CECT for staging of DA specifically. In 1992 Kazerooni published a study about CT for duodenal neoplasms in 25 patients. However, only eight patients with DA were included and the study mainly focused on differentiating benign from malignant neoplasms [41]. Furthermore, we included patients with all stages of DA, in contrary to many other studies where only resected patients, which are the minority, are included. Including all stages allows for better generalization of results for all DA patients. A limitation is the retrospective nature of the study and a long time range of included patients, which led to a variation of CECT protocols. However, 75% of the CECT scans dated after 2015, the scan year and the availability of an HAP did not seem to influence the performance for staging, but the groups were small (data not shown). Furthermore, variation in scanning protocols is a reflection of daily clinical practice.
Conclusion
Our study shows that routine CECT is limited for staging and determining resectability in patients with duodenal adenocarcinoma. In 19% of patients, the tumor could not be resected due to unexpected local vessel invasion and/or metastases. Radiologists and clinicians have to be aware that staging of DA with CECT is inadequate, for that reason additional imaging techniques are necessary. This could include CT or MRI with hypotonic duodenography, DWI-MR, USPIO-enhanced MRI, 18FDG-PET-CT, or 68 Ga-FAPI-PET-CT. Based on the knowledge and experience obtained with this study, we would advise a multiphase hypotonic duodenography CT combined with an MRI scan as the most optimal workup for DA. We implemented this strategy in our hospital for future patients with (suspected) DA. However, this strategy has not been adequately investigated and therefore a multicenter trial is needed to prove the validity of this imaging strategy.
Abbreviations
- CECT:
-
Contrast-enhanced computed tomography
- CHA:
-
Common hepatic artery
- DA:
-
Duodenal adenocarcinoma
- DP:
-
Delayed phase
- DPCG:
-
Dutch Pancreatic Cancer Group
- DWI:
-
Diffusion-weighted imaging
- EUS:
-
Endoscopic ultrasound
- 18FDG-PET-CT:
-
Fluorine-18-fluorodeoxyglucose positron emission tomography computed tomography
- 68 Ga-FAPI-PET-CT:
-
Gallium-68-conjugated FAP inhibitor positron emission tomography computed tomography
- HAP:
-
Hepatic arterial phase
- LN:
-
Lymph node
- MRI:
-
Magnetic resonance imaging
- OS:
-
Overall survival
- TNM:
-
Tumor node metastases
- USPIO:
-
Ultra-small superparamagnetic iron oxide
- PVP:
-
Portal-venous phase
- SMA:
-
Superior mesenteric artery
- SMV:
-
Superior mesenteric vein
References
Netherlands Cancer Registry (NCR). Netherlands Comprehensive Cancer Organisation (IKNL) available via https://www.iknl.nl/en/ncr. Accessed 14 July 2021.
Legue LM, Bernards N, Gerritse SL et al (2016) Trends in incidence, treatment and survival of small bowel adenocarcinomas between 1999 and 2013: a population-based study in The Netherlands. Acta Oncol 55:1183-1189. Doi:https://doi.org/10.1080/0284186X.2016.1182211
Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J (2004) Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 101:518-526. Doi:https://doi.org/10.1002/cncr.20404
Greenlee RT, Murray T, Bolden S, Wingo PA (2000) Cancer statistics, 2000. CA Cancer J Clin 50:7-33. Doi:https://doi.org/10.3322/canjclin.50.1.7
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7-30. Doi:https://doi.org/10.3322/caac.21590
Akce M, Jiang R, Zakka K et al (2019) Clinical Outcomes of Small Bowel Adenocarcinoma. Clin Colorectal Cancer 18:257-268. Doi:https://doi.org/10.1016/j.clcc.2019.08.002
Halfdanarson TR, McWilliams RR, Donohue JH, Quevedo JF (2010) A single-institution experience with 491 cases of small bowel adenocarcinoma. Am J Surg 199:797-803. Doi:https://doi.org/10.1016/j.amjsurg.2009.05.037
Tian J, Liu J, Guo C et al (2019) Prognostic factors and treatment outcomes in patients with non-ampullary small bowel adenocarcinoma: Long-term analysis. Medicine (Baltimore) 98:e15381. Doi:https://doi.org/10.1097/MD.0000000000015381
Aparicio T, Zaanan A, Svrcek M et al (2014) Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis 46:97-104. Doi:https://doi.org/10.1016/j.dld.2013.04.013
Amr B, Miles G, Shahtahmassebi G, Roobottom C, Stell DA (2017) Systematic evaluation of radiological findings in the assessment of resectability of peri-ampullary cancer by CT using different contrast phase protocols. Clin Radiol 72:691 e611–691 e617. Doi:https://doi.org/10.1016/j.crad.2017.02.012
Beenen E, van Roest MH, Sieders E et al (2014) Staging laparoscopy in patients scheduled for pancreaticoduodenectomy minimizes hospitalization in the remaining life time when metastatic carcinoma is found. Eur J Surg Oncol 40:989-994. Doi:https://doi.org/10.1016/j.ejso.2013.12.019
Huprich JE, Fletcher JG, Fidler JL et al (2011) Prospective blinded comparison of wireless capsule endoscopy and multiphase CT enterography in obscure gastrointestinal bleeding. Radiology 260:744-751. Doi:https://doi.org/10.1148/radiol.11110143
Meijer LL, Alberga AJ, de Bakker JK et al (2018) Outcomes and Treatment Options for Duodenal Adenocarcinoma: A Systematic Review and Meta-Analysis. Ann Surg Oncol 25:2681-2692. Doi:https://doi.org/10.1245/s10434-018-6567-6
Cloyd JM, George E, Visser BC (2016) Duodenal adenocarcinoma: Advances in diagnosis and surgical management. World J Gastrointest Surg 8:212-221. Doi:https://doi.org/10.4240/wjgs.v8.i3.212
Li Y, Wang J, Ma X et al (2016) A Review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Int J Biol Sci 12:1022-1031. Doi:https://doi.org/10.7150/ijbs.15438
Eyck BM, van der Wilk BJ, Lagarde SM et al (2018) Neoadjuvant chemoradiotherapy for resectable oesophageal cancer. Best Pract Res Clin Gastroenterol 36-37:37-44. Doi:https://doi.org/10.1016/j.bpg.2018.11.007
Sakae H, Kanzaki H, Nasu J et al (2017) The characteristics and outcomes of small bowel adenocarcinoma: a multicentre retrospective observational study. Br J Cancer 117:1607-1613. Doi:https://doi.org/10.1038/bjc.2017.338
Cecchini S, Correa-Gallego C, Desphande V et al (2012) Superior prognostic importance of perineural invasion vs. lymph node involvement after curative resection of duodenal adenocarcinoma. J Gastrointest Surg 16:113–120; discussion 120. Doi:https://doi.org/10.1007/s11605-011-1704-6
Locher C, Batumona B, Afchain P et al (2018) Small bowel adenocarcinoma: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis 50:15-19. Doi:https://doi.org/10.1016/j.dld.2017.09.123
Benson AB, Venook AP, Al-Hawary MM et al (2020) Small Bowel Adenocarcinoma, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network (NCCN)
AJCC (2018) AJCC Cancer Staging Manual, Eighth Edition.
www.dpcg.nl PREOPANC trial: definitions of resectability.
Brooks AD, Mallis MJ, Brennan MF, Conlon KC (2002) The value of laparoscopy in the management of ampullary, duodenal, and distal bile duct tumors. J Gastrointest Surg 6:139–145; discussion 145–136. Doi:https://doi.org/10.1016/s1091-255x(01)00021-x
Stell D, Mayer D, Mirza D, Buckels J (2004) Delayed diagnosis and lower resection rate of adenocarcinoma of the distal duodenum. Dig Surg 21:434–438; discussion 438–439. Doi:https://doi.org/10.1159/000083470
Fong ZV, Tan WP, Lavu H et al (2013) Preoperative imaging for resectable periampullary cancer: clinicopathologic implications of reported radiographic findings. J Gastrointest Surg 17:1098-1106. Doi:https://doi.org/10.1007/s11605-013-2181-x
Park HS, Lee JM, Kim SH et al (2010) Three-dimensional MDCT for preoperative local staging of gastric cancer using gas and water distention methods: a retrospective cohort study. AJR Am J Roentgenol 195:1316-1323. Doi:https://doi.org/10.2214/AJR.10.4320
Hwang SW, Lee DH (2014) Is endoscopic ultrasonography still the modality of choice in preoperative staging of gastric cancer? World J Gastroenterol 20:13775-13782. Doi:https://doi.org/10.3748/wjg.v20.i38.13775
Cronin CG, Dowd G, Mhuircheartaigh JN et al (2009) Hypotonic MR duodenography with water ingestion alone: feasibility and technique. Eur Radiol 19:1731-1735. Doi:https://doi.org/10.1007/s00330-009-1346-1
Masselli G, Di Tola M, Casciani E et al (2016) Diagnosis of Small-Bowel Diseases: Prospective Comparison of Multi-Detector Row CT Enterography with MR Enterography. Radiology 279:420-431. Doi:https://doi.org/10.1148/radiol.2015150263
Cheng SM, Ling W, Zhu J, Xu JR, Wu LM, Gong HX (2018) Dual Energy Spectral CT Imaging in the assessment of Gastric Cancer and cell proliferation: A Preliminary Study. Sci Rep 8:17619. Doi:https://doi.org/10.1038/s41598-018-35712-w
Wei W, Yu Y, Lv W, Deng K, Yuan L, Zhao Y (2014) Predictive value of dual-energy spectral computed tomographic imaging on the histological origin of carcinomas in the ampullary region. Abdom Imaging 39:702-710. Doi:https://doi.org/10.1007/s00261-014-0098-9
Fortuin AS, Bruggemann R, van der Linden J et al (2018) Ultra-small superparamagnetic iron oxides for metastatic lymph node detection: back on the block. Wiley Interdiscip Rev Nanomed Nanobiotechnol 10. Doi:https://doi.org/10.1002/wnan.1471
Nakai G, Matsuki M, Harada T et al (2011) Evaluation of axillary lymph nodes by diffusion-weighted MRI using ultrasmall superparamagnetic iron oxide in patients with breast cancer: initial clinical experience. J Magn Reson Imaging 34:557-562. Doi:https://doi.org/10.1002/jmri.22651
Koh DM, Collins DJ, Wallace T, Chau I, Riddell AM (2012) Combining diffusion-weighted MRI with Gd-EOB-DTPA-enhanced MRI improves the detection of colorectal liver metastases. Br J Radiol 85:980-989. Doi:https://doi.org/10.1259/bjr/91771639
Watanabe N, Hayashi S, Kato H et al (2004) FDG-PET imaging in duodenal cancer. Ann Nucl Med 18:351-353. Doi:https://doi.org/10.1007/BF02984475
Sperti C, Pasquali C, Fiore V et al (2006) Clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the management of patients with nonpancreatic periampullary neoplasms. Am J Surg 191:743-748. Doi:https://doi.org/10.1016/j.amjsurg.2005.03.042
Pang Y, Zhao L, Luo Z et al (2021) Comparison of (68)Ga-FAPI and (18)F-FDG Uptake in Gastric, Duodenal, and Colorectal Cancers. Radiology 298:393-402. Doi:https://doi.org/10.1148/radiol.2020203275
Lee TC, Wima K, Morris MC et al (2020) Small Bowel Adenocarcinomas: Impact of Location on Survival. J Surg Res 252:116-124. Doi:https://doi.org/10.1016/j.jss.2020.03.017
Oweira H, Abdel-Rahman O, Mehrabi A, Reissfelder C (2019) Assessment of the external validity of the AJCC 8(th) staging system for small intestinal adenocarcinoma: a time to reconsider the role of tumor location? J Gastrointest Oncol 10:421-428. Doi:https://doi.org/10.21037/jgo.2019.01.15
Wilhelm A, Galata C, Beutner U et al (2018) Duodenal localization is a negative predictor of survival after small bowel adenocarcinoma resection: A population-based, propensity score-matched analysis. J Surg Oncol 117:397-408. Doi:https://doi.org/10.1002/jso.24877
Kazerooni EA, Quint LE, Francis IR (1992) Duodenal neoplasms: predictive value of CT for determining malignancy and tumor resectability. AJR Am J Roentgenol 159:303-309. Doi:https://doi.org/10.2214/ajr.159.2.1632344
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by G. Litjens, J.J. Hermans, and C.J.H.M. van Laarhoven. The first draft of the manuscript was written by G. Litjens and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ethical approval
Institutional Review Board approval was obtained.
Consent to participate
Written informed consent was waived by the Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Litjens, G., van Laarhoven, C.J.H.M., Prokop, M. et al. Routine contrast-enhanced CT is insufficient for TNM-staging of duodenal adenocarcinoma. Abdom Radiol 47, 3436–3445 (2022). https://doi.org/10.1007/s00261-022-03589-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03589-z