Abstract
Purpose
To evaluate the feasibility of Quantitative Ultrashort-Time-to-Echo Contrast-Enhanced (QUTE-CE) MRA using ferumoxytol as a contrast agent for abdominal angiography in the kidney.
Methods
Four subjects underwent ferumoxytol-enhanced MRA with the 3D UTE Spiral VIBE WIP sequence at 3 T. Image quality metrics were quantified, specifically the blood Signal-to-Noise Ratio (SNR), blood-tissue Contrast-to-Noise Ratio (CNR) and Intraluminal Signal Heterogeneity (ISH) from both the aorta and inferior vena cava (IVC). Morphometric analysis of the vessels was performed using manual approach and semi-automatic approach using Vascular Modeling ToolKit (VMTK). Image quality and branching order were compared between QUTE-CE MRA and the Gadolinium (Gd) CEMRA reference image.
Results
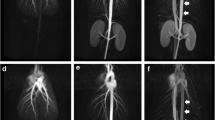
QUTE-CE MRA provides a bright blood snapshot that delineates arteries and veins equally in the same scan. The maximum SNR and CNR values were 3,282 ± 1,218 and 1,295 ± 580, respectively – significantly higher than available literature values using other CEMRA techniques. QUTE-CE MRA had lower ISH and depicted higher vessel branching order (7th vs 3rd) within the kidney compared to a standard dynamic clinical Gd CEMRA scan. Morphometric analysis yielded quantitative results for the total kidney volume, total cyst volume and for diameters of the branching arterial network down to the 7th branch. Vessel curvature was significantly increased (p < 0.001) in the presence of a renal cyst compared to equivalent vessels in normal kidney regions.
Conclusion
QUTE-CE MRA is feasible for kidney angiography, providing greater detail of kidney vasculature, enabling quantitative morphometric analysis of the abdominal and intra-renal vessels and yielding metrics relevant to vascular diseases while using a contrast agent ferumoxytol that is safe for CKD patients.






Similar content being viewed by others
Availability of data and material
All data analyzed during this study were included in this manuscript and its supplementary information.
Code availability
Advanced Normalization Toolkit (ANTS, http://stnava.github.io/ANTs/), 3D Slicer (http://www.slicer.org), Vascular Modeling ToolKit (VMTK, www.vmtk.org).
References
Murphy, D., et al., Trends in Prevalence of Chronic Kidney Disease in the United States. Ann Intern Med, 2016. 165(7): p. 473-481.
Waikar, S.S., et al., Biological Variability of Estimated GFR and Albuminuria in CKD. Am J Kidney Dis, 2018. 72(4): p. 538-546.
Selby, N.M., et al., Magnetic resonance imaging biomarkers for chronic kidney disease: a position paper from the European Cooperation in Science and Technology Action PARENCHIMA. Nephrol Dial Transplant, 2018. 33(suppl_2): p. ii4-ii14.
Bellasi, A. and P. Raggi, Vascular imaging in chronic kidney disease. Curr Opin Nephrol Hypertens, 2012. 21(4): p. 382-8.
Josephs, S.C., Accuracy of Computed Tomographic Angiography and Magnetic Resonance Angiography for Diagnosing Renal Artery Stenosis. Perspectives in Vascular Surgery and Endovascular Therapy, 2005. 17(2): p. 180-182.
Perazella, M.A., Advanced kidney disease, gadolinium and nephrogenic systemic fibrosis: the perfect storm. Curr Opin Nephrol Hypertens, 2009. 18(6): p. 519-25.
Schieda, N., et al., Gadolinium-Based Contrast Agents in Kidney Disease: Comprehensive Review and Clinical Practice Guideline Issued by the Canadian Association of Radiologists. Can Assoc Radiol J, 2018. 69(2): p. 136-150.
Fotenos, A. Update on FDA approach to safety issue of gadolinium retention after administration of gadolinium-based contrast agents 2018; Available from: https://www.fda.gov/media/116492/download.
Mahan Mathur, M.W., MD. Patient evaluation before gadolinium contrast administration for magnetic resonance imaging. 2020; Available from: https://www.uptodate.com/contents/patient-evaluation-before-gadolinium-contrast-administration-for-magnetic-resonance-imaging#H1951507250.
Bongartz, G., M. Mayr, and D. Bilecen, Magnetic resonance angiography (MRA) in renally impaired patients: when and how. Eur J Radiol, 2008. 66(2): p. 213-9.
Mukundan, S., et al., Ferumoxytol-Enhanced Magnetic Resonance Imaging in Late-Stage CKD. Am J Kidney Dis, 2016. 67(6): p. 984-8.
Edelman, R.R. and I. Koktzoglou, Noncontrast MR angiography: An update. J Magn Reson Imaging, 2019. 49(2): p. 355-373.
Neuwelt, E.A., et al., Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int, 2009. 75(5): p. 465-74.
Luhar, A., et al., Contrast-enhanced magnetic resonance venography in pediatric patients with chronic kidney disease: initial experience with ferumoxytol. Pediatr Radiol, 2016. 46(9): p. 1332-40.
Nguyen, K.L., et al., Ferumoxytol-enhanced MR Angiography for Vascular Access Mapping before Transcatheter Aortic Valve Replacement in Patients with Renal Impairment: A Step Toward Patient-specific Care. Radiology, 2018. 286(1): p. 326-337.
Toth, G.B., et al., Current and potential imaging applications of ferumoxytol for magnetic resonance imaging, in Kidney International. 2017. p. 47–66.
Wells, S.A., et al., Pharmacokinetics of Ferumoxytol in the Abdomen and Pelvis: A Dosing Study with 1.5- and 3.0-T MRI Relaxometry. Radiology, 2019: p. 190489.
Aime, S. and P. Caravan, Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging, 2009. 30(6): p. 1259-67.
Theruvath, A.J., et al., Brain iron deposition after Ferumoxytol-enhanced MRI: A study of Porcine Brains. Nanotheranostics, 2020. 4(4): p. 195-200.
Stuber, M., et al., Positive contrast visualization of iron oxide-labeled stem cells using inversion-recovery with ON-resonant water suppression (IRON). Magn Reson Med, 2007. 58(5): p. 1072-7.
Gharagouzloo, C.A., et al., Quantitative vascular neuroimaging of the rat brain using superparamagnetic nanoparticles: New insights on vascular organization and brain function. Neuroimage, 2017. 163: p. 24-33.
Knobloch, G., et al., Comparison of gadolinium-enhanced and ferumoxytol-enhanced conventional and UTE-MRA for the depiction of the pulmonary vasculature. Magn Reson Med, 2019. 82(5): p. 1660-1670.
Tustison, N.J., et al., N4ITK: improved N3 bias correction. IEEE Trans Med Imaging, 2010. 29(6): p. 1310-20.
Avants, B., N. Tustison, and G. Song, Advanced Normalization Tools (ANTS). Insight Journal, 2009. 2(365): p. 1-35.
Fedorov, A., et al., 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging, 2012. 30(9): p. 1323-41.
Sigovan, M., et al., USPIO-enhanced MR angiography of arteriovenous fistulas in patients with renal failure. Radiology, 2012. 265(2): p. 584-590.
Li, Y., et al., The flatness index of inferior vena cava is useful in predicting hypovolemic shock in severe multiple-injury patients. J Emerg Med, 2013. 45(6): p. 872-8.
Gharagouzloo, C.A., P.N. McMahon, and S. Sridhar, Quantitative contrast-enhanced MRI with superparamagnetic nanoparticles using ultrashort time-to-echo pulse sequences. Magn Reson Med, 2015. 74(2): p. 431-41.
Bak, S.H., et al., Appropriate Minimal Dose of Gadobutrol for 3D Time-Resolved MRA of the Supra-Aortic Arteries: Comparison with Conventional Single-Phase High-Resolution 3D Contrast-Enhanced MRA. AJNR Am J Neuroradiol, 2017. 38(7): p. 1383-1390.
Dehkharghani, S., et al., Dose Reduction in Contrast-Enhanced Cervical MR Angiography: Field Strength Dependency of Vascular Signal Intensity, Contrast Administration, and Arteriographic Quality. AJR Am J Roentgenol, 2015. 204(6): p. W701-6.
Nael, K., et al., Combined low-dose contrast-enhanced MR angiography and perfusion for acute ischemic stroke at 3T: A more efficient stroke protocol. AJNR Am J Neuroradiol, 2014. 35(6): p. 1078-84.
Hansmann, J., et al., Enhancement characteristics and impact on image quality of two gadolinium chelates at equimolar doses for time-resolved 3-Tesla MR-angiography of the calf station. PLoS One, 2014. 9(6): p. e99079.
Kramer, J.H., et al., Dynamic and static magnetic resonance angiography of the supra-aortic vessels at 3.0 T: intraindividual comparison of gadobutrol, gadobenate dimeglumine, and gadoterate meglumine at equimolar dose. Invest Radiol, 2013. 48(3): p. 121–8.
Haneder, S., et al., Comparison of 0.5 M gadoterate and 1.0 M gadobutrol in peripheral MRA: a prospective, single-center, randomized, crossover, double-blind study. J Magn Reson Imaging, 2012. 36(5): p. 1213–21.
Raman, F.S., et al., 3.0-T whole-heart coronary magnetic resonance angiography: comparison of gadobenate dimeglumine and gadofosveset trisodium. Int J Cardiovasc Imaging, 2013. 29(5): p. 1085–94.
Schabel, M.C. and D.L. Parker, Uncertainty and bias in contrast concentration measurements using spoiled gradient echo pulse sequences. Phys Med Biol, 2008. 53(9): p. 2345-73.
Nayak, A.B., et al., High-resolution, whole-body vascular imaging with ferumoxytol as an alternative to gadolinium agents in a pediatric chronic kidney disease cohort. Pediatr Nephrol, 2015. 30(3): p. 515-21.
Schubert, T., et al., Crossover comparison of ferumoxytol and gadobenate dimeglumine for abdominal MR-angiography at 3.0 tesla: Effects of contrast bolus length and flip angle. J Magn Reson Imaging, 2017. 45(6): p. 1617–1626.
Gameraddin, M., Normal abdominal aorta diameter on abdominal sonography in healthy asymptomatic adults: impact of age and gender. Journal of Radiation Research and Applied Sciences, 2019. 12(1): p. 186-191.
François, C.J., et al., Renal Arteries: Isotropic, High-Spatial-Resolution, Unenhanced MR Angiography with Three-dimensional Radial Phase Contrast. Radiology, 2011. 258(1): p. 254-260.
Fabrega-Foster, K.E., et al., Efficacy and safety of gadobutrol-enhanced MRA of the renal arteries: Results from GRAMS (Gadobutrol-enhanced renal artery MRA study), a prospective, intraindividual multicenter phase 3 blinded study. J Magn Reson Imaging, 2018. 47(2): p. 572-581.
Liang, K.W., et al., The Performance of Noncontrast Magnetic Resonance Angiography in Detecting Renal Artery Stenosis as Compared With Contrast Enhanced Magnetic Resonance Angiography Using Conventional Angiography as a Reference. J Comput Assist Tomogr, 2017. 41(4): p. 619-627.
Bello-Reuss, E., K. Holubec, and S. Rajaraman, Angiogenesis in autosomal-dominant polycystic kidney disease. Kidney Int, 2001. 60(1): p. 37-45.
Ruangwattanapaisarn, N., A. Hsiao, and S.S. Vasanawala, Ferumoxytol as an off-label contrast agent in body 3T MR angiography: a pilot study in children. Pediatr Radiol, 2015. 45(6): p. 831-9.
Aja-Fernandez, S., G. Vegas-Sanchez-Ferrero, and A. Tristan-Vega, Noise estimation in parallel MRI: GRAPPA and SENSE. Magn Reson Imaging, 2014. 32(3): p. 281-90.
Gong, I.H., et al., Relationship among total kidney volume, renal function and age. J Urol, 2012. 187(1): p. 344-9.
Acknowledgments
We acknowledge Dr. John E. Kirsch for helpful advice on experimental setup.
Funding
This study was funded by National Institutes of Health (Grant numbers 1R41DA043974-01 and 1R21DK118449-01).
Author information
Authors and Affiliations
Contributions
LT, MH and SS contributed to the study concept and design. LT, JQ, RML, VM, AA, GVJ and MH contributed to data acquisition. Data analysis were performed by LT, TZ and YL. The manuscript was drafted by LT, TZ and SS. Critical editing and revision of the manuscript was done by LT, TZ, SS, RTS, and DD. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
RTS reports personal fees from Siemens Medical Solutions, Inc., outside the submitted work. SS reports grants from Theranano LLC and grants from Northeastern University, during the conduct of the study; In addition, SS has a patent WO2017019182A1 pending.
Ethical approval
This study was conducted with approval by the Massachusetts General Hospital Institutional Review board IRB:2017P001262. All procedures performed in the study involving human participants were Heal Insurance Portability and Accountability Act (HIPAA) compliant.
Consent to participate
Subjects provided informed written consent for the imaging procedure which is an off-label use of ferumoxytol.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Electronic supplementary material 2 (MP4 5980 kb)
Rights and permissions
About this article
Cite this article
Timms, L., Zhou, T., Lyu, Y. et al. Ferumoxytol-enhanced ultrashort TE MRA and quantitative morphometry of the human kidney vasculature. Abdom Radiol 46, 3288–3300 (2021). https://doi.org/10.1007/s00261-021-02984-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-02984-2