Abstract
Purpose
To investigate the added value of gadoxetic acid-enhanced MRI in monitoring liver function and disease progression in patients with primary sclerosing cholangitis (PSC).
Methods
We retrospectively identified 104 consecutive patients (75 males; mean age 41.98 ± 12.5 years) with confirmed diagnosis of PSC who underwent 227 gadoxetic acid-enhanced MRI examinations between January 2008 and May 2019. Relative enhancement (RE) of the liver was correlated with the results of liver function tests (LFTs), scoring models (Model for End-Stage Liver Disease (MELD) score, Mayo Risk Score (MRS), Amsterdam-Oxford model (AOM)), and qualitative MRI findings. In addition, results were analyzed separately for excretory MRI examinations (n = 164) and nonexcretory examinations (n = 63) depending on excretion of gadoxetic acid into the common bile duct in the hepatobiliary phase (HBP).
Results
There was a significant correlation of RE with MRS (r = − 0.652), MELD score (r = − 0.474), AOM (r = − 0.468), and LFTs (P < 0.001). RE and albumin were significantly higher in the excretory group whereas scoring models, bilirubin, aspartate aminotransferase, alkaline phosphatase, and international normalized ratio were lower (P < 0.001). RE was lower in segments with absent HBP gadoxetic acid excretion into dilated bile ducts, reduced HBP parenchymal enhancement, atrophy, T2 hyperintensity, and bile duct abnormalities (P < 0.001).
Conclusion
Relative enhancement of the liver in gadoxetic acid-enhanced MRI can be used to evaluate global and regional liver function and monitor disease progression in patients with PSC. Hepatobiliary phase gadoxetic acid biliary excretion appears to be a reproducible qualitative parameter for evaluating disease severity that can be easily integrated into routine clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary sclerosing cholangitis (PSC) is a rare, chronic cholestatic liver disease of unknown etiology that is characterized by progressive diffuse inflammation, obliterating fibrosis, stricture formation, and destruction of the intra- and extrahepatic bile ducts and will ultimately progress to liver cirrhosis and end-stage liver disease [1,2,3].
Different clinical, laboratory, histologic, and cholangiographic scoring models have been developed to estimate disease severity and predict the clinical course of PSC and patient outcome. Other potential applications of these prognostic scoring models include prediction of the response to therapy and risk stratification following therapeutic interventions [4,5,6,7]. The Mayo risk score (MRS) was designed specifically for assessing the short-term (4-year) mortality risk of PSC patients. However, the MRS was developed in a group of patients with end-stage liver disease and is not suitable for use in early PSC. In addition, it cannot predict survival of individual patients [4, 5, 8,9,10]. The Amsterdam cholangiographic classification system is limited clinically by its invasive nature [11, 12]. The Model for End-Stage Liver Disease (MELD) score is a valid prognostic score for prediction of both the short- and intermediate-term mortality risk of patients with chronic liver disease [13,14,15]. The Amsterdam-Oxford model (AOM), the most recently recommended prognostic model, can predict long-term transplant-free survival in PSC patients [4, 16].
Because of its invasiveness and associated complications, endoscopic retrograde cholangiopancreatography (ERCP) is now restricted to therapeutic interventions in patients with PSC. Magnetic resonance imaging (MRI), including magnetic resonance cholangiopancreatography (MRCP), has replaced ERCP as the imaging modality of choice for diagnosis of PSC [6, 17, 18].
Gadoxetic acid is a hepatocyte-specific contrast agent that allows not only morphological but also functional evaluation of global and regional liver and consequently can be used as a prognostic marker in patients with PSC [5, 19]. Biliary excretion, which represents around 50% of gadoxetic acid excretion in individuals with normal liver and kidney function, is delayed in patients with impaired liver function and biliary obstruction [20, 21].
The purpose of our study is to investigate gadoxetic acid-enhanced MRI as a surrogate imaging-based model for evaluation of liver function and disease progression in patients with PSC, focusing on relative enhancement (RE) of the liver and gadoxetic acid biliary excretion in the hepatobiliary phase (HBP).
Materials and methods
Patient population and study design
We retrospectively identified 126 consecutive patients with confirmed diagnosis of PSC who underwent gadoxetic acid-enhanced MRI examinations (n = 268) at our institution between January 2008 and May 2019 from the picture archiving and communication system (PACS) and patients’ electronic medical records. The diagnosis of PSC was established in accordance with the guidelines of the European Association for the Study of the Liver (EASL) [17, 22]. This retrospective study was approved by the institutional review board. Informed consent was waived.
Inclusion criteria were a confirmed diagnosis of PSC according to EASL guidelines and completion of the MRI examination (including MRCP). Exclusion criteria were: history of cholangiocarcinoma, liver transplantation (LTx), liver resection or locoregional liver intervention for management of hepatic malignancy, and nondiagnostic image quality due to severe artifacts.
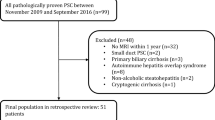
After exclusion, 104 patients who underwent 227 MRI examinations remained for analysis (Fig. 1).
Laboratory parameters and clinical scoring systems
Liver function tests (LFTs) (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase [ALP], gamma-glutamyl transferase [GGT], serum total bilirubin, and serum albumin), kidney function tests (serum creatinine and estimated glomerular filtration rate [eGFR]), international normalized ratio (INR), platelets, C-reactive protein (CRP), and white blood cell (WBC) count performed within 1 month before or after gadoxetic acid-enhanced MRI were selected for analysis. In addition, tumor marker (carbohydrate antigen 19-9 [CA 19-9]) was tested in patients with suspected cholangiocarcinoma.
The MELD score (n = 207) (based on serum bilirubin, creatinine, and INR) [13], MRS (n = 96) (based on patient age, bilirubin, albumin, AST, and history of variceal bleeding) [8], and AOM (n = 92) (based on patient age at diagnosis, PSC subtype [large-duct–small-duct], bilirubin, albumin, AST, ALP, and platelet count) [16] including 5-, 10- and 15-year transplant-free estimated survival rates were prospectively calculated using data collected from patients’ electronic medical records.
MRI examinations
MRI examinations were performed at our institution using 6 different scanners: 1.5T Magnetom Avanto, 1.5T Magnetom Aera, 3.0T Magnetom Skyra, 3.0T Biograph mMR (Siemens Healthcare, Erlangen, Germany), 1.5T Intera (Philips, Best, The Netherlands), and 1.5T GE Signa Excite (GE Medical Systems, Milwaukee, WI, USA). In all examinations, transverse T1-weighted images covering the entire liver with 60–80 slices were acquired before and approximately 20 min after intravenous bolus injection of 0.1 ml/kg body weight of gadoxetic acid (Gd-EOB-DTPA, gadoxetate disodium; Primovist®/Eovist®, Bayer HealthCare, Berlin, Germany). MRCP and T2-weighted sequences were performed before gadoxetic acid administration. Sequence parameters are listed in Supplementary Table 1.
Image analysis
All MRI examinations were reviewed by two reader with 8 and 12 years of experience in abdominal imaging and MRI who were blinded to clinical data and laboratory findings.
Quantitative analysis
Images were analyzed using a dedicated viewing workstation (Centricity PACS RA1000 version 6.0, General Electric, Milwaukee, WI, USA). Signal intensity (SI) was measured by placing one circular region of interest (ROI) approximately 2.5 cm in diameter in each Couinaud liver segment (8 ROIs). Each ROI was placed in identical locations in images acquired prior to (SI unenhanced) and approximately 20 min after gadoxetic acid administration in the HBP (SI in HBP). Large vessels (caliber > 5 mm), bile ducts, tumor masses, and artifacts were avoided. RE during the HBP was calculated for each segment and the whole liver (i.e., mean SI of the 8 measurements) using the following formula:
Qualitative analysis
Each liver segment was evaluated for the following imaging features: 1-degree of bile duct dilatation, 2-significant bile duct stenosis, 3-bile duct caliber irregularity including beading (multiple segmental caliber irregularities in the form of strictures alternating with dilatations) and pruning (peripheral bile duct attenuations) [23], 4-isolated alterations in parenchymal signal intensity (T2 hyperintensity), 5-egmental lobar atrophy, 6-HBP parenchymal contrast enhancement and 7-HBP gadoxetic acid excretion into dilated segmental bile ducts (Supplementary Table 2).
Relative enhancement was correlated with scoring results (MELD score, MRS, and AOM) and different laboratory values. At the segmental level, RE was correlated with different qualitative imaging findings.
MRI examinations were graded as excretory (164 examinations) versus nonexcretory (63 examinations) depending on HBP biliary excretion of gadoxetic acid into the common bile duct (CBD). These two subgroups were compared regarding RE, results of prognostic scoring models, and laboratory values.
In a subgroup analysis of patients who underwent at least two gadoxetic acid-enhanced MRI examinations, HBP gadoxetic acid biliary excretion into the CBD was evaluated as a predictor of liver function and disease severity based on the changes in RE, scoring results and LFTs (bilirubin, ALP, albumin, INR).
Statistical analysis
Bivariate analysis (Pearson correlation) was used for simple correlation analysis. The paired t test and Mann–Whitney U-test were performed to assess differences between two groups. Linear mixed model analysis was used to identify possible predictors of gadoxetic acid biliary excretion during the HBP. Repeated-measures analysis was performed using a linear mixed model. Receiver operating characteristic (ROC) analysis was used to identify cutoff values for different parameters (RE, scoring models, bilirubin, albumin, and ALP) for visualization of HBP gadoxetic acid biliary excretion into the CBD. Statistical analysis was performed with Stata/MP version 16.0 (StataCorp, College Station, Texas, USA). A P value < 0.05 was considered statistically significant.
Results
Study population
The study included 104 patients (75 males, 29 females; mean age 41.98 ± 12.5 years, age range 15.8–78 years). They had a mean age of 34.4 ± 12.4 years when diagnosed with PSC. Apart from 2 patients (who underwent 3 MRI scans) with small-duct PSC, all patients had large-duct PSC. Patient demographics are presented in Table 1.
Analysis of SI and RE of the liver
Mean SI of the liver was 214.32 ± 6.7 before administration of gadoxetic acid (SI unenhanced) and 342.07 ± 12.4 in the HBP. Mean RE was 0.57 ± 0.02. On a lobar level, RE was significantly higher in the right lobe (0.59 ± 0.02) than in the left lobe (0.55 ± 0.02, P < 0.001). Patients with PSC complicated by liver cirrhosis (108/227) had significantly lower RE than patients without cirrhosis (0.51 ± 0.02 vs. 0.62 ± 0.02, P < 0.001). Analysis of RE in the HBP in relation to the amount of gadoxetic acid administered revealed a significant positive correlation (r = 0.156, P = 0.019). Supplementary Table 3 provides descriptive results of SI and RE analysis.
Clinical scores and laboratory findings
With an AOM of 2.05 ± 0.79, the study population had a considerable risk for LTx or death, while a MRS of 0.52 ± 1.43 and a MELD score of 9 indicated an intermediate risk.
Correlation analysis between RE of the whole liver and different clinical scores revealed a significant negative correlation (P < 0.001) with MRS (r = − 0.652), MELD score (r = − 0.474), and AOM (r = − 0.468) (Fig. 2) and significant positive correlation (P < 0.001) with 5-year, 10-year and 15-year transplant-free survival rates in AOM (Table 2). There was a significant negative correlation between RE and LFTs apart from serum albumin, for which there was a significant positive correlation. (Table 2).
Pearson correlation of mean relative enhancement (RE) of the liver in the hepatobiliary phase with clinical scores. Scatterplot with regression line demonstrates a significant negative correlation (P < 0.001) of RE with a Model for End-Stage Liver Disease (MELD) score, b Mayo risk score, and c Amsterdam-Oxford model
Evaluation of gadoxetic acid biliary excretion
Regarding the excretion of gadoxetic acid into the CBD in the HBP (Fig. 3), the excretory group had a significantly higher RE and a significantly lower MRS, MELD score, and AOM (P < 0.001). Comparison of LFT results between the excretory and nonexcretory group revealed significantly higher levels of serum bilirubin (P < 0.001), ALP (P < 0.001), AST(P = 0.002), and INR (P = 0.001) in the nonexcretory group and a significantly higher level of serum albumin (P < 0.001) in the excretory group (Table 3).
T2-weighted sequence (HASTE) (a1, b1, c1), MRCP (a2, b2, c2), and T1-weighted VIBE sequence 20 min after gadoxetic acid administration (in the HBP) (axial sequence: a3, b3, b3 and coronal sequence: a4, b4, c4) in three patients with different stages of PSC. Patient a: Early PSC changes with peripheral bile duct attenuation and slight T2 hyperintensity in the right posterior liver segment. Gadoxetic acid-enhanced images in the HBP demonstrate homogenous parenchymal enhancement and regular excretion of gadoxetic acid in the CBD. Patient b: Typical advanced PSC changes with focal impairment of liver parenchyma in the form of significant segmental bile duct stenosis, T2 hyperintensity, and atrophy affecting most liver segments apart from segments V and VIII. Gadoxetic acid-enhanced images in the HBP demonstrate heterogeneous parenchyma with absent enhancement in affected segments and absent excretion of gadoxetic acid in the CBD. Patient c: End-stage PSC reflected by cirrhotic liver configuration, multisegment bile duct stenosis and atrophy, as well as absent contrast enhancement and excretion of gadoxetic acid in the CBD in the HBP. CBD common bile duct; HASTE half-Fourier acquisition single-shot turbo spin-echo; VIBE volumetric interpolated breath-hold sequence; HBP hepatobiliary phase; PSC primary sclerosing cholangitis
ROC analysis was performed to identify the parameter most useful for predicting HBP gadoxetic acid biliary excretion into the CBD. Statistical significance was noted for RE, scoring models, and LFTs (bilirubin, albumin, ALP). Relative enhancement had the largest AUC of 0.880 (P < 0.001). A cutoff value of 0.43 had 85.9% sensitivity and 80.6% specificity for the presence of HBP gadoxetic acid excretion (Fig. 4, Table 4).
At the segmental level, analysis of HBP gadoxetic acid excretion into dilated segmental bile ducts revealed significantly higher RE in segments demonstrating contrast excretion (0.68 ± 0.29) than in segments without excretion (0.56 ± 0.31, P < 0.001) (Table 5).
Analysis of qualitative imaging findings
Analysis of various qualitative MRI findings by liver segment showed RE in the HBP to be significantly higher in segments without bile duct dilatation (0.61 ± 0.34) than in segments with minimal (0.52 ± 0.31, P < 0.001), mild (0.42 ± 0.29, P < 0.001), or marked bile duct dilatation (0.35 ± 0.29, P < 0.001). However, when comparing RE between segments with mild and marked bile duct dilatation, we noted no significant difference (P = 0.13). Similarly, RE was significantly lower in segments demonstrating bile duct caliber irregularity, significant bile duct stenosis, atrophy, reduced HBP parenchymal enhancement, or T2 hyperintensity (P < 0.001) than in segments without those findings (Table 5).
The results of correlation of RE with qualitative imaging findings at the segmental level are presented in Table 6. The highest correlation was observed with segmental bile duct dilatation (6/8 segments) and HBP gadoxetic acid excretion into dilated segmental bile ducts (5/8 segments), while the lowest correlation was noted with segmental atrophy (1/8 segments).
Patients with at least two gadoxetic acid-enhanced MRI examinations
Subgroup linear mixed model analysis of patients who underwent at least two gadoxetic acid-enhanced MRI examinations revealed that HBP gadoxetic acid biliary excretion into the CBD was a significant predictor of liver function and disease severity based on the prediction of changes in RE, scoring results (MELD score, MRS, and AOM), and LFTs (bilirubin, albumin, INR) (P < 0.001) (Table 7).
In addition, repeated-measures analysis using linear mixed model comparison revealed that RE tended to gradually decrease with increasing scores and bilirubin levels over a longer period of time (Fig. 5). Statistically significant difference in RE was noted between first and second MRI examinations (P = 0.03) and this significance was higher in the excretory group (P = 0.001) (Supplementary Table 4). Results of descriptive analysis of patients with at least two gadoxetic acid-enhanced MRI examinations are listed in Supplementary Table 5.
Discussion
Since MRI/MRCP emerged as the modality of choice in the diagnosis and follow-up of patients with PSC, there has been an ongoing interest in developing an MRI-based surrogate parameter for the noninvasive evaluation of disease progression in patients with PSC [24].
The present study investigated gadoxetic acid-enhanced MRI-derived quantitative and qualitative parameters—specifically RE of the liver and gadoxetic acid biliary excretion during the HBP, respectively—as imaging-based surrogate parameters for liver function evaluation (global and regional) and disease progression in patients with PSC.
Relative enhancement of the liver in the HBP correlated significantly with the MRS (moderate correlation), MELD score, and the AOM (low correlation). In addition, RE correlated significantly with LFTs including the suggested surrogate endpoints (bilirubin, ALP, albumin, and INR) [25]. This significant correlation with the scoring models and surrogate endpoints supports the potential of RE as a surrogate prognostic parameter for evaluation of global liver function as well as prediction of short-, intermediate-, and long-term survival.
These findings are consistent with previous studies investigating liver function evaluation in patients with PSC using gadoxetic acid-enhanced MRI-derived indices. Schulze et al. demonstrated a moderate correlation of RE with prognostic scoring models (MELD, MRS, AOM) and LFTs (ALP, albumin, bilirubin, INR). They proposed a RE cutoff of 0.65 for prediction of clinical endpoints with 73.86% sensitivity and 92.86% specificity [26].
Nilsson et al. demonstrated a significant correlation of the hepatic extraction fraction (HEF), input relative blood-flow (irBF), and mean transit time (MTT) with the MRS [5]. Contrary to our results, there was no significant correlation with the MELD score. The most probable explanation might be that they only analyzed a small sample including 12 PSC patients who had predominantly mild disease. Hinrichs et al. investigated T1-mapping in gadoxetic acid-enhanced MRI for evaluating global and regional liver function. They demonstrated a significant correlation of a shorter T1 relaxation time with the MRS, MELD score, AST, bilirubin, and cholinesterase [19].
In the present study, we evaluated the added value of HBP gadoxetic acid biliary excretion into the CBD and/or the duodenum as a qualitative imaging parameter. The group with gadoxetic acid biliary excretion had significantly higher RE, longer estimated transplant-free survival, lower scores in clinical scoring models, and lower levels in LFTs (apart from albumin, which was significantly higher). These findings are in agreement with previous studies performed by Ringe et al. [21] and Nolz et al. [23]. Bastati et al. retrospectively evaluated functional liver imaging score (FLIS) derived from gadoxetic acid-enhanced MRI for estimation of liver function and prediction of transplant-free survival in patients with chronic liver disease. Biliary excretion in the HBP was one of the three FLIS parameters. They found the FLIS to be an independent risk factor for the first hepatic decompensation and mortality [27].
Also, to our knowledge, ours is the first study that analyzed the consistency of liver function over time in PSC patients with at least two gadoxetic acid-enhanced MRI examinations. The analysis revealed HBP gadoxetic acid excretion to be a significant predictor of temporal changes of RE, scoring models, and LFTs (bilirubin, albumin, INR). These findings support the value of HBP gadoxetic acid biliary excretion as a marker of disease severity in patients with PSC.
All of the previously investigated scoring models, surrogate endpoints, and LFTs have only been validated for assessing global liver function, which is not optimal in patients with PSC, which is characterized by heterogeneous distribution of liver function and severity of disease progression. Regional liver function evaluation is paramount for the detection of early PSC, assessment for resectability in patients with cholangiocarcinoma, and for identifying severely affected segments for endoscopic guided drainage or targeting biopsies to reduce sampling errors especially in patients with suspected small-duct PSC [19, 26, 28].
Several findings of the present study provide evidence that segmental liver function in PSC patients can be estimated using RE in gadoxetic acid-enhanced MRI such as the significant difference in RE between right and left hepatic lobes (which could be due to the higher incidence of left lateral segmental atrophy and the effect of gravity which could resulted in a relatively higher enhancement in the right posterior liver segments) and the significantly lower RE in segments with impaired liver parenchyma (no gadoxetic acid excretion within the dilated bile ducts, reduced HBP parenchymal enhancement, atrophy, T2 hyperintensity) and bile duct abnormalities (irregularities, dilatation, significant stenosis).
In addition, these findings support our hypothesis that qualitative findings in gadoxetic acid-enhanced MRI can assist in identifying segments that may benefit from targeted stenting and separate them from segments with already lost function where intervention is not justified. We conclude that the absence of HBP gadoxetic acid excretion into dilated segmental bile ducts combined with the absence of parenchymal enhancement and segmental atrophy indicates segments for which targeted stenting is not advisable and could even be hazardous considering the higher risk of cholangitis associated with retained contrast medium since these segments are not excreting. In contrast, targeted stenting is advised for segments demonstrating gadoxetic acid excretion into the dilated bile ducts, parenchymal enhancement, and no segmental atrophy in the HBP in order to prevent further deterioration of segmental function and thus improve overall liver function capacity. A scoring model including RE, HBP gadoxetic acid biliary excretion, and variable qualitative findings—specifically, bile duct abnormalities—can be used for risk stratification and prediction of disease severity in PSC patients. This approach deserves further investigation in future studies.
Our study has several limitations First, we used a retrospective study design. Second, there was no correlation with clinical endpoints such as LTx or death. Third, there might be bias from the use of different MRI scanners and different field strengths. Fourth, we only evaluated gadoxetic acid biliary excretion in the HBP approximately 20 min after gadoxetic acid administration. A second, delayed HBP acquisition at 30–60 min would have added to the validity of our findings considering the well-known fact that HBP excretion of gadoxetic acid is reduced in patients with chronic liver disease. Such a delayed acquisition was previously suggested by Ringe et al. [21] Fifth, we did not correlate the qualitative findings of bile duct abnormalities in T2-weighted MRI/MRCP with ERCP findings or the previously suggested Amsterdam cholangiographic score. We did not consider the latter because it is not widely validated and only takes bile duct abnormalities but not parenchymal changes into account. Sixth, there was no reference standard against which to correlate regional hepatic gadoxetic acid-enhanced MRI measurements. We correlated RE with several qualitative MRI findings. However, the qualitative nature makes them prone to interindividual variation. Finally, in the repeated-measures analysis, and due to the retrospective nature of the study, the exact time point of MRI acquisition was not standardized, which could be another possible cause of bias.
In conclusion, relative enhancement of the liver during the hepatobiliary phase in gadoxetic acid-enhanced MRI can be used to evaluate global and regional liver function and monitor disease progression in patients with PSC. Hepatobiliary phase gadoxetic acid biliary excretion appears to be a reproducible qualitative parameter for evaluating disease severity that can be easily integrated into routine clinical practice.
Data availability
Data and materials used during conducting the study are available. (data transparency).
Change history
04 July 2021
The following OA funding note is addressed above reference section: Open Access funding enabled and organized by Projekt DEAL.
05 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00261-021-03155-z
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- CBD:
-
Common bile duct
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- GGT:
-
Gamma-glutamyl transferase
- HBP:
-
Hepatobiliary phase
- INR:
-
International normalized ratio
- LFT:
-
Liver function test
- MELD:
-
Model for End-Stage Liver Disease
- MRS:
-
Mayo Risk Score
- PSC:
-
Primary sclerosing cholangitis
- RE:
-
Relative enhancement
- SI:
-
Signal intensity
References
R. Chapman, J. Fevery, A. Kalloo, D.M. Nagorney, K.M. Boberg, B. Shneider, G.J. Gores, D. American Association for the Study of Liver, Diagnosis and management of primary sclerosing cholangitis, Hepatology 51(2) (2010) 660-78. https://doi.org/10.1002/hep.23294.
J.H. Tabibian, C.L. Bowlus, Primary sclerosing cholangitis: A review and update, Liver Res 1(4) (2017) 221-230. https://doi.org/10.1016/j.livres.2017.12.002.
T.H. Karlsen, T. Folseraas, D. Thorburn, M. Vesterhus, Primary sclerosing cholangitis - a comprehensive review, J Hepatol 67(6) (2017) 1298-1323. https://doi.org/10.1016/j.jhep.2017.07.022.
J.C. Goet, A. Floreani, X. Verhelst, N. Cazzagon, L. Perini, W.J. Lammers, A.C. de Vries, A.J. van der Meer, H.R. van Buuren, B.E. Hansen, Validation, clinical utility and limitations of the Amsterdam-Oxford model for primary sclerosing cholangitis, J Hepatol 71(5) (2019) 992-999. https://doi.org/10.1016/j.jhep.2019.06.012.
H. Nilsson, L. Blomqvist, L. Douglas, A. Nordell, H. Jacobsson, K. Hagen, A. Bergquist, E. Jonas, Dynamic gadoxetate-enhanced MRI for the assessment of total and segmental liver function and volume in primary sclerosing cholangitis, J Magn Reson Imaging 39(4) (2014) 879-886. https://doi.org/10.1002/jmri.24250.
S. Lemoinne, N. Cazzagon, S. El Mouhadi, P.J. Trivedi, A. Dohan, A. Kemgang, K. Ben Belkacem, C. Housset, Y. Chretien, C. Corpechot, G. Hirschfield, A. Floreani, R. Motta, B. Gallix, A. Barkun, J. Barkun, O. Chazouilleres, L. Arrive, Simple Magnetic Resonance Scores Associate With Outcomes of Patients With Primary Sclerosing Cholangitis, Clin Gastroenterol Hepatol (2019). https://doi.org/10.1016/j.cgh.2019.03.013.
E.M. de Vries, M. de Krijger, M. Farkkila, J. Arola, P. Schirmacher, D. Gotthardt, B. Goeppert, P.J. Trivedi, G.M. Hirschfield, H. Ytting, B. Vainer, H.R. Buuren, K. Biermann, M.H. Harms, O. Chazouilleres, D. Wendum, A.D. Kemgang, R.W. Chapman, L.M. Wang, K.D. Williamson, A.S. Gouw, V. Paradis, C. Sempoux, U. Beuers, S.G. Hubscher, J. Verheij, C.Y. Ponsioen, Validation of the prognostic value of histologic scoring systems in primary sclerosing cholangitis: An international cohort study, Hepatology 65(3) (2017) 907-919. https://doi.org/10.1002/hep.28963.
W.R. Kim, T.M. Therneau, R.H. Wiesner, J.J. Poterucha, J.T. Benson, M. Malinchoc, N.F. LaRusso, K.D. Lindor, E.R. Dickson, A revised natural history model for primary sclerosing cholangitis, Mayo Clin Proc 75(7) (2000) 688-94. https://doi.org/10.4065/75.7.688.
P.J. Trivedi, Risk stratification in primary sclerosing cholangitis: It’s time to move on from replicating imperfection and break the glass ceiling, J Hepatol 71(5) (2019) 867-870. https://doi.org/10.1016/j.jhep.2019.08.013.
E. Christensen, Prognostic models including the Child–Pugh, MELD and Mayo risk scores—where are we and where should we go?, J Hepatol 41(2) (2004) 344-350. https://doi.org/10.1016/j.jhep.2004.06.005.
C.Y. Ponsioen, S.M. Vrouenraets, W. Prawirodirdjo, R. Rajaram, E.A. Rauws, C.J. Mulder, J.B. Reitsma, S.H. Heisterkamp, G.N. Tytgat, Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population, Gut 51(4) (2002) 562-6. https://doi.org/10.1136/gut.51.4.562.
C.Y. Ponsioen, J.B. Reitsma, K.M. Boberg, L. Aabakken, E.A.J. Rauws, E. Schrumpf, Validation of a cholangiographic prognostic model in primary sclerosing cholangitis, Endoscopy 42(09) (2010) 742-747. https://doi.org/10.1055/s-0030-1255527.
P.S. Kamath, R.H. Wiesner, M. Malinchoc, W. Kremers, T.M. Therneau, C.L. Kosberg, G. D’Amico, E.R. Dickson, W.R. Kim, A model to predict survival in patients with end-stage liver disease, Hepatology 33(2) (2001) 464-70. https://doi.org/10.1053/jhep.2001.22172.
. Said, J. Williams, J. Holden, P. Remington, R. Gangnon, A. Musat, M.R. Lucey, Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease, J Hepatol 40(6) (2004) 897-903. https://doi.org/10.1016/j.jhep.2004.02.010.
F. Botta, E. Giannini, P. Romagnoli, A. Fasoli, F. Malfatti, B. Chiarbonello, E. Testa, D. Risso, G. Colla, R. Testa, MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study, Gut 52(1) (2003) 134-139. https://doi.org/10.1136/gut.52.1.134.
E.M. de Vries, J. Wang, K.D. Williamson, M.M. Leeflang, K. Boonstra, R.K. Weersma, U. Beuers, R.W. Chapman, R.B. Geskus, C.Y. Ponsioen, A novel prognostic model for transplant-free survival in primary sclerosing cholangitis, Gut 67(10) (2018) 1864-1869. https://doi.org/10.1136/gutjnl-2016-313681.
L. Aabakken, T.H. Karlsen, J. Albert, M. Arvanitakis, O. Chazouilleres, J.-M. Dumonceau, M. Färkkilä, P. Fickert, G.M. Hirschfield, A. Laghi, M. Marzioni, M. Fernandez, S.P. Pereira, J. Pohl, J.-W. Poley, C.Y. Ponsioen, C. Schramm, F. Swahn, A. Tringali, C. Hassan, Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline, J Hepatol 66(6) (2017) 1265-1281. https://doi.org/10.1016/j.jhep.2017.02.013.
N. Cazzagon, O. Chazouillères, C. Corpechot, S. El Mouhadi, E. Chambenois, B. Desaint, U. Chaput, S. Lemoinne, L. Arrivé, Predictive criteria of response to endoscopic treatment for severe strictures in primary sclerosing cholangitis, Clin Res Hepatol Gastroenterol 43(4) (2019) 387-394. https://doi.org/10.1016/j.clinre.2019.01.003.
H. Hinrichs, J.B. Hinrichs, M. Gutberlet, H. Lenzen, H.J. Raatschen, F. Wacker, K.I. Ringe, Functional gadoxetate disodium-enhanced MRI in patients with primary sclerosing cholangitis (PSC), Eur Radiol 26(4) (2016) 1116-24. https://doi.org/10.1007/s00330-015-3913-y.
C. Schramm, J. Eaton, K.I. Ringe, S. Venkatesh, J. Yamamura, M.R.I.w.g.o.t. IPSCSG, Recommendations on the use of magnetic resonance imaging in PSC-A position statement from the International PSC Study Group, Hepatology (Baltimore, Md.) 66(5) (2017) 1675-1688. https://doi.org/10.1002/hep.29293.
K.I. Ringe, J. Hinrichs, E.M. Merkle, T.J. Weismüller, F. Wacker, B.C. Meyer, Gadoxetate disodium in patients with primary sclerosing cholangitis: an analysis of hepatobiliary contrast excretion, J Magn Reson Imaging 40(1) (2014) 106-12. https://doi.org/10.1002/jmri.24381.
L. European Association for the Study of the, EASL Clinical Practice Guidelines: management of cholestatic liver diseases, J Hepatol 51(2) (2009) 237-267. https://doi.org/10.1016/j.jhep.2009.04.009.
R. Nolz, U. Asenbaum, M. Schoder, A. Wibmer, H. Einspieler, A.M. Prusa, M. Peck-Radosavljevic, A. Ba-Ssalamah, Diagnostic workup of primary sclerosing cholangitis: the benefit of adding gadoxetic acid-enhanced T1-weighted magnetic resonance cholangiography to conventional T2-weighted magnetic resonance cholangiography, Clin Radiol 69(5) (2014) 499-508. https://doi.org/10.1016/j.crad.2013.12.008.
E.A. Selvaraj, E.L. Culver, H. Bungay, A. Bailey, R.W. Chapman, M. Pavlides, Evolving role of magnetic resonance techniques in primary sclerosing cholangitis, World J Gastroenterol 25(6) (2019) 644-658. https://doi.org/10.3748/wjg.v25.i6.644.
T. Watanabe, K. Hirano, M. Tada, H. Isayama, S. Mizuno, T. Arizumi, N. Toda, Y. Sugawara, N. Kokudo, K. Koike, Short-term prognostic factors for primary sclerosing cholangitis, J Hepatobiliary Pancreat Sci 22(6) (2015) 486-490. https://doi.org/10.1002/jhbp.238.
J. Schulze, H. Lenzen, J.B. Hinrichs, B. Ringe, M.P. Manns, F. Wacker, K.I. Ringe, An Imaging Biomarker for Assessing Hepatic Function in Patients With Primary Sclerosing Cholangitis, Clin Gastroenterol Hepatol 17(1) (2019) 192-199.e3. https://doi.org/10.1016/j.cgh.2018.05.011.
N. Bastati, L. Beer, M. Mandorfer, S. Poetter-Lang, D. Tamandl, Y. Bican, M.C. Elmer, H. Einspieler, G. Semmler, B. Simbrunner, M. Weber, J.C. Hodge, F. Vernuccio, C. Sirlin, T. Reiberger, A. Ba-Ssalamah, Does the Functional Liver Imaging Score Derived from Gadoxetic Acid-enhanced MRI Predict Outcomes in Chronic Liver Disease?, Radiology 294(1) (2020) 98-107. https://doi.org/10.1148/radiol.2019190734.
S. Keller, S.K. Venkatesh, M. Avanesov, J.M. Weinrich, R. Zenouzi, C. Schramm, G. Adam, J. Yamamura, Gadolinium-based relative contrast enhancement in primary sclerosing cholangitis: additional benefit for clinicians?, Clin Radiol 73(7) (2018) 677.e1-677.e6. https://doi.org/10.1016/j.crad.2018.02.010.
Acknowledgements
The authors thank Bettina Herwig for language editing.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Each author has participated sufficiently in this submission to take public responsibility for its content.
Corresponding author
Ethics declarations
Conflict of interest
PD Dr. med. Dominik Geisel and Prof. Dr. med. Timm Denecke have received travel grant and honoraria from Bayer AG. Beyond that there is nothing to disclose.
Ethical approval
The study was approved by the IRB. Informed consent was waived by the IRB.
Consent to participate
Each author has participated sufficiently in this submission to take public responsibility for its content.
Consent for publication
The publication was approved by all authors and the responsible authorities where the work was carried out.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to a retrospective open access order.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elkilany, A., Geisel, D., Müller, T. et al. Gadoxetic acid-enhanced MRI in primary sclerosing cholangitis: added value in assessing liver function and monitoring disease progression. Abdom Radiol 46, 979–991 (2021). https://doi.org/10.1007/s00261-020-02731-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02731-z