Abstract
Purpose
To elucidate the relationships between mural nodules (MNs) and invasive components in patients with invasive intraductal papillary mucinous neoplasm (IPMN) on the basis of thin-section contrast-enhanced multidetector CT (CE-MDCT) and pathologic findings.
Methods
This retrospective study included 28 patients with surgically confirmed invasive IPMN. Two radiologists independently evaluated the thin-section (1-mm section thickness, no overlap) triple-phase CE-MDCT images for MNs, invasive components, and the continuity between them using a five-point scale (confidence scores of 1–3 as negative, 4 and 5 as positive). Kappa statistic was used to evaluate interobserver agreement. The CE-MDCT findings were correlated with pathologic findings.
Results
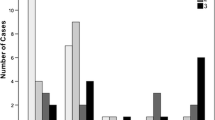
Interobserver agreement was good or excellent. MNs consisting of tumor cells were recognized in 12 (42.9%) of 28 patients with no discrepancy between the two radiologists. Invasive components were detected in 85.7% and 82.1% in the pancreatic parenchymal phase for radiologist 1 and 2, respectively, and recognized as hypoattenuating areas. Pathologic continuities between MNs and invasive components were confirmed in five (41.7%) of 12 patients with MNs and these were detected on CE-MDCT. When combined seven patients without continuities between MNs and invasive components and 16 patients without MNs, the invasive components pathologically derived from non-nodular low-height papillary epithelium in 23 (82.1%) of 28 patients.
Conclusions
The invasive components derived more often from low-height papillary epithelium without MN appearance on CE-MDCT than from MN. Careful attention should be paid to the existence of an invasive component even in the absence of an enhancing MN.




Similar content being viewed by others
Abbreviations
- EUS:
-
Endoscopic ultrasonography
- MDCT:
-
Multidetector computed tomography
- CE:
-
Contrast enhanced
- IPMN:
-
Intraductal papillary mucinous neoplasm
- MN:
-
Mural nodule
- MPD:
-
Main pancreatic duct
References
Hruban RH, Takaori K, Klimstra DS et al. (2004) An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 28:977-987.
Sohn TA, Yeo CJ, Cameron JL et al. (2004) Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg 239:788-797.
Adsay, N.V., Fukushima, N, Furukawa, T. et al., Intraductal neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. (2010) WHO classification of tumours of the digestive system: Lyon: WHO Press. pp 304-313.
Doi R, Fujimoto K, Wada M, Imamura M (2002) Surgical management of intraductal papillary mucinous tumor of the pancreas. Surgery 132:80-85.
Nara S, Shimada K, Kosuge T, Kanai Y, Hiraoka N (2008) Minimally invasive intraductal papillary-mucinous carcinoma of the pancreas: clinicopathologic study of 104 intraductal papillary-mucinous neoplasms. Am J Surg Pathol 32:243-255.
Kang MJ, Lee KB, Jang JY et al. (2013) Disease spectrum of intraductal papillary mucinous neoplasm with an associated invasive carcinoma: invasive IPMN versus pancreatic ductal adenocarcinoma-associated IPMN. Pancreas 42:1267-1274.
Tian X, Gao H, Ma Y, Zhuang Y, Yang Y (2015) Surgical treatment and prognosis of 96 cases of intraductal papillary mucinous neoplasms of the pancreas: a retrospective cohort study. Int J Surg 13:49-53.
Tanaka M, Fernández-del Castillo C, Adsay V et al. (2012) International Consensus Guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12:183-197.
Seo N, Byun JH, Kim JH et al. (2016) Validation of the 2012 International Consensus Guidelines using computed tomography and magnetic resonance imaging. Ann Surg 263:557-564.
Shimizu Y, Yamaue H, Maguchi H et al. (2013) Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas 42:883-888.
Tanaka M, Fernández-Del Castillo C, Kamisawa T, et al. (2017) Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 5:738-753.
European Study Group on Cystic Tumours of the Pancreas (2018) European evidence-based guidelines on pancreatic cystic neoplasms. Gut 67:789-804.
Kawada N, Uehara H, Nagata S, Tsuchishima M, Tsutsumi M, Tomita Y (2016) Mural nodule of 10 mm or larger as predictor of malignancy for intraductal papillary mucinous neoplasm of the pancreas: Pathologic and radiological evaluations. Pancreatology 16:441-448.
Pedrosa I, Boparai D (2010) Imaging considerations in intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg 27:324-330.
Nakagawa A, Yamaguchi T, Ohtsuka M, et al. (2009) Usefulness of multidetector computed tomography for detecting protruding lesions in intraductal papillary mucinous neoplasm of the pancreas in comparison with single-detector computed tomography and endoscopic ultrasonography. Pancreas 38:131-136.
Choi SY, Kim JH, Yu MH, Eun HW, Lee HK, Han JK (2017) Diagnostic performance and imaging features for predicting the malignant potential of intraductal papillary mucinous neoplasm of the pancreas: a comparison of EUS, contrast-enhanced CT and MRI. Abdom Radiol (NY) 42:1449-1458.
Kang HJ, Lee JM, Joo I, et al. (2016) Assessment of Malignant Potential in Intraductal Papillary Mucinous Neoplasms of the Pancreas: Comparison between Multidetector CT and MR Imaging with MR Cholangiopancreatography. Radiology 279:128-139.
Vullierme MP, Giraud-Cohen M, Hammel P, et al. (2007) Malignant intraductal papillary mucinous neoplasm of the pancreas: in situ versus invasive carcinoma surgical resectability. Radiology 245:483-490.
Manfredi R, Graziani R, Motton M, et al. (2009) Main pancreatic duct intraductal papillary mucinous neoplasms: accuracy of MR imaging in differentiation between benign and malignant tumors compared with histopathologic analysis. Radiology 253:106-115.
Ogawa H, Itoh S, Ikeda M, Suzuki K, Naganawa S (2008) Intraductal papillary mucinous neoplasm of the pancreas: assessment of the likelihood of invasiveness with multisection CT. Radiology 248:876-886.
Yamada Y, Mori H, Matsumoto S, Kiyosue H, Hori Y, Hongo N (2010) Pancreatic adenocarcinoma versus chronic pancreatitis: differentiation with triple-phase helical CT. Abdom Imaging 35:163-171.
Furuhashi N, Suzuki K, Sakurai Y, Ikeda M, Kawai Y (2015) Naganawa S, Differentiation of focal-type autoimmune pancreatitis from pancreatic carcinoma: assessment by multiphase contrast-enhanced CT. Eur Radiol 25:1366-1374.
Tamada T, Ito K, Kanomata N et al. (2016) Pancreatic adenocarcinomas without secondary signs on multiphasic multidetector CT: association with clinical and histopathologic features. Eur Radiol 26:646-655.
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159-174.
Koshita S, Fujita N, Noda Y et al. (2015) Invasive carcinoma derived from "flat type" branch duct intraductal papillary mucinous neoplasms of the pancreas: impact of classification according to the height of mural nodule on endoscopic ultrasonography. J Hepatobiliary Pancreat Sci 22:301-309.
Kim JH, Eun HW, Kim KW et al. (2013) Intraductal papillary mucinous neoplasms with associated invasive carcinoma of the pancreas: imaging findings and diagnostic performance of MDCT for prediction of prognostic factors. AJR Am J Roentgenol 201:565-572.
Yamada Y, Mori H, Matsumoto S, Hijiya N, Hongo N, Moriyama M (2010) Invasive carcinomas originating from intraductal papillary mucinous neoplasms of the pancreas: conspicuity and primary sites of the solid masses on triple-phase dynamic CT imaging. Abdom Imaging 35:181-188.
Hattori YI, Gabata T, Matsui O et al. (2009) Enhancement patterns of pancreatic adenocarcinoma on conventional dynamic multi-detector row CT: correlation with angiogenesis and fibrosis. World J Gastroenterol 15:3114-3131.
Vege SS, Ziring B, Jain R, Moayyedi P (2015); Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 148:819-822.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamei, N., Yamada, Y., Hijiya, N. et al. Invasive intraductal papillary mucinous neoplasms of the pancreas: relationships between mural nodules detected on thin-section contrast-enhanced MDCT and invasive components. Abdom Radiol 44, 3139–3147 (2019). https://doi.org/10.1007/s00261-019-02084-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02084-2