Abstract
Objective
To apply texture analysis (TA) in computed tomography (CT) of urinary stones and to correlate TA findings with the number of required shockwaves for successful shock wave lithotripsy (SWL).
Materials and methods
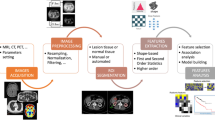
CT was performed on thirty-four urinary stones in an in vitro setting. Urinary stones underwent SWL and the number of required shockwaves for disintegration was recorded. TA was performed after post-processing for pixel spacing and image normalization. Feature selection and dimension reduction were performed according to inter- and intrareader reproducibility and by evaluating the predictive ability of the number of shock waves with the degree of redundancy between TA features. Three regression models were tested: (1) linear regression with elimination of colinear attributes (2), sequential minimal optimization regression (SMOreg) employing machine learning, and (3) simple linear regression model of a single TA feature with lowest squared error.
Results
Highest correlations with the absolute number of required SWL shockwaves were found for the linear regression model (r = 0.55, p = 0.005) using two weighted TA features: Histogram 10th Percentile, and Gray-Level Co-Occurrence Matrix (GLCM) S(3, 3) SumAverg. Using the median number of required shockwaves (n = 72) as a threshold, receiver-operating characteristic analysis showed largest area-under-the-curve values for the SMOreg model (AUC = 0.84, r = 0.51, p < 0.001) using four weighted TA features: Histogram 10th Percentile, and GLCM S(1, 1) InvDfMom, S(3, 3) SumAverg, and S(4, −4) SumVarnc.
Conclusion
Our in vitro study illustrates the proof-of-principle of TA of urinary stone CT images for predicting the success of stone disintegration with SWL.


Similar content being viewed by others
References
Pearle MS (2012) Shock-wave lithotripsy for renal calculi. N Engl J Med 367(1):50–57
Scales CD, et al. (2012) Prevalence of kidney stones in the United States. Eur Urol 62(1):160–165
Stamatelou KK, et al. (2003) Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 63(5):1817–1823
Assimos D, et al. (2016) Surgical management of stones: American urological association/endourological society guideline. PART II. J Urol 196(4):1161–1169
Turk C, et al. (2016) EAU guidelines on interventional treatment for urolithiasis. Eur Urol 69(3):475–482
Koo V, et al. (2011) Cost-effectiveness and efficiency of shockwave lithotripsy vs flexible ureteroscopic holmium:yttrium-aluminium-garnet laser lithotripsy in the treatment of lower pole renal calculi. BJU Int 108(11):1913–1916
Albala DM, et al. (2001) Lower pole I: a prospective randomized trial of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy for lower pole nephrolithiasis-initial results. J Urol 166(6):2072–2080
Macaluso JN Jr, Thomas R (1991) Extracorporeal shock wave lithotripsy: an outpatient procedure. J Urol 146(3):714–717
Mandhani A, et al. (2003) Prediction of fragility of urinary calculi by dual X-ray absorptiometry. J Urol 170(4 Pt 1):1097–1100
Lindqvist K, et al. (2006) Extracorporeal shock-wave lithotripsy or ureteroscopy as primary treatment for ureteric stones: a retrospective study comparing two different treatment strategies. Scand J Urol Nephrol 40(2):113–118
Pareek G, et al. (2005) Extracorporeal shock wave lithotripsy success based on body mass index and Hounsfield units. Urology 65(1):33–36
Williams, J.C., Jr., et al., Variability of renal stone fragility in shock wave lithotripsy. Urology, 2003. 61(6): p. 1092–6; discussion 1097.
Stolzmann P, et al. (2010) In vivo identification of uric acid stones with dual-energy CT: diagnostic performance evaluation in patients. Abdom Imaging 35(5):629–635
Hameed DA, et al. (2013) Comparing non contrast computerized tomography criteria versus dual X-ray absorptiometry as predictors of radio-opaque upper urinary tract stone fragmentation after electromagnetic shockwave lithotripsy. Urolithiasis 41(6):511–515
Ng CF, et al. (2009) Development of a scoring system from noncontrast computerized tomography measurements to improve the selection of upper ureteral stone for extracorporeal shock wave lithotripsy. J Urol 181(3):1151–1157
Tanaka M, et al. (2013) Stone attenuation value and cross-sectional area on computed tomography predict the success of shock wave lithotripsy. Korean J Urol 54(7):454–459
El-Nahas, A.R., et al., A prospective multivariate analysis of factors predicting stone disintegration by extracorporeal shock wave lithotripsy: the value of high-resolution noncontrast computed tomography. Eur Urol, 2007. 51(6): p. 1688–93; discussion 1693–4.
Largo R, et al. (2016) Predictive value of low tube voltage and dual-energy CT for successful shock wave lithotripsy: an in vitro study. Urolithiasis 44(3):271–276
Mullhaupt G, et al. (2015) How do stone attenuation and skin-to-stone distance in computed tomography influence the performance of shock wave lithotripsy in ureteral stone disease? BMC Urol 15:72
Aerts HJ, et al. (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006
Summers RM (2017) Texture analysis in radiology: Does the emperor have no clothes? Abdom Radiol (NY) 42(2):342–345
Kassner A, et al. (2009) Prediction of hemorrhagic transformation in acute ischemic stroke using texture analysis of postcontrast T1-weighted MR images. J Magn Reson Imaging 30(5):933–941
Mouthuy N, et al. (2012) Multiparametric magnetic resonance imaging to differentiate high-grade gliomas and brain metastases. J Neuroradiol 39(5):301–307
Zhang, G.M., et al., Differentiating pheochromocytoma from lipid-poor adrenocortical adenoma by CT texture analysis: feasibility study. Abdom Radiol (NY), 2017.
Zhang GM, et al. (2017) Quantitative CT texture analysis for evaluating histologic grade of urothelial carcinoma. Abdom Radiol (NY) 42(2):561–568
Takahashi N, et al. (2016) CT negative attenuation pixel distribution and texture analysis for detection of fat in small angiomyolipoma on unenhanced CT. Abdom Radiol (NY) 41(6):1142–1151
Yu H, et al., Texture analysis as a radiomic marker for differentiating renal tumors. Abdom Radiol (NY), 2017.
Szczypiński PM, et al. (2009) MaZda–a software package for image texture analysis. Comput Methods Progr Biomed 94(1):66–76
Collewet G, Strzelecki M, Mariette F (2004) Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging 22(1):81–91
Tahir F, Fahiem MA (2014) A statistical-textural-features based approach for classification of solid drugs using surface microscopic images. Comput Math Methods Med 2014:791246
Szczypiński, PM, et al. (2009) MaZda—the software package for textural analysis of biomedical images, vol. 65. New York: Springer
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Sogawa, K., et al., Neurogenic and myogenic diseases: quantitative texture analysis of muscle US data for differentiation. Radiology, 2017: p. 160826.
Kotrotsou A, Zinn PO, Colen RR (2016) Radiomics in brain tumors: an emerging technique for characterization of tumor environment. Magn Reson Imaging Clin N Am 24(4):719–729
Hayano K, et al. (2016) Exploration of imaging biomarkers for predicting survival of patients with advanced non-small cell lung cancer treated with antiangiogenic chemotherapy. AJR 206(5):987–993
Lubner MG, et al. (2017) Texture analysis of the liver at MDCT for assessing hepatic fibrosis. Abdom Radiol (NY) 1:1–10
Rozenberg R, et al. (2016) Whole-tumor quantitative apparent diffusion coefficient histogram and texture analysis to predict Gleason score upgrading in intermediate-risk 3 + 4 = 7 Prostate Cancer. AJR 206(4):775–782
Shevade SK, et al. (2000) Improvements to the SMO algorithm for SVM regression. IEEE Trans Neural Netw 11(5):1188–1193
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was received for this study.
Conflict of interest
None of the authors has any conflict of interest to declare.
Ethical approval
Local ethics committee approval was not required for this phantom study.
Rights and permissions
About this article
Cite this article
Mannil, M., von Spiczak, J., Hermanns, T. et al. Prediction of successful shock wave lithotripsy with CT: a phantom study using texture analysis. Abdom Radiol 43, 1432–1438 (2018). https://doi.org/10.1007/s00261-017-1309-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1309-y